The viral vector market is highly active, and the interest in production technologies is driven by recent approvals in cell and gene therapy, says Ã…sa Hagner-McWhirter, senior scientist at Cytiva. Demand for viral vector production is high. The main driver is the large pipeline in different clinical stages and scales. In 2018 there were 724 clinical trials for gene therapies and gene-modified cell therapies, many of which use viral vectors. Adenoviruses and retroviruses, including lentivirus, are frequently used. Adeno-associated virus…

Upstream & Downstream Processing
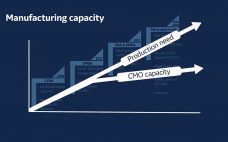
Prefab facilities could feed the need for COVID vaccine capacity, say Univercells and Exyte
Exyte and Univercells Technologies have teamed to develop and construct modular GMP vaccine manufacturing plants that can be deployed in a third of the time of conventional facilities. As vaccines against COVID-19, caused by the SARS-CoV-2 virus, begin to move through the clinic at an unprecedented speed, the question is rising as to how industry can scale-up production. At the heart of this is ensuring capacity is available to produce millions, if not billions, of doses of a potential vaccine.…
Cytiva to help Takara gear up for COVID-19 vaccine production
Cytiva will help Japan’s Takara Bio prepare manufacturing processes for a DNA-based SARS-CoV-2 vaccine candidate. Cytiva announced the collaboration this week, explaining it will work with Takara and partners Osaka University and AnGes to develop production processes and technology for the vaccine. Stephane Perrey, general manager for Cytiva in Japan, Australia, and New Zealand, told us “Cytiva is providing process design consultation, prioritization of equipment manufacturing and prioritization of consumables manufacturing. “We worked closely with Takara Bio to help them…
Rapid Micro Biosystems to fund EU facility construction with $120m fundraising
US sterility testing tech firm Rapid Micro Biosystems will build a European plant and expand its business with $120 million raised this week. The Lowell, Massachusetts firm – which makes automated microbial detection systems – completed a $120 million financing round this week, attracting equity investment from The Ally Bridge Group, Endeavour Vision, and existing investors including Asahi Kasei. CEO Robert Spignesi told Bioprocess Insider the firm’s “growth plan includes establishing new manufacturing capacity, beyond our current US manufacturing facility…
MilliporeSigma reports robust Q1 on back of COVID-19
Merck’s MilliporeSigma is the latest to report bioprocess resilience in the face of COVID-19, and like others has even seen an increase in sales and projects. MilliporeSigma, the life sciences division of Germany’s Merck, saw sales of €1.8 billion ($2 billion) in the first quarter of 2020, up 6.5% on the same period last year, fueled in part by its Process Solutions business unit, which saw sales grow organically by 13.2% (another example of double-digit growth in the biopharma equipment…
Greenlight raises $17m for mRNA vaccine capacity hike citing COVID-19 target
GreenLight Biosciences says a manufacturing expansion will allow it to make billions of doses of its mRNA-based COVID-19 vaccine. The Boston biotech announced the expansion plan last week, explaining it will use $17 million raised in a recent funding round to build out its scalable mRNA production capability. GreenLight is developing several mRNA vaccine candidates against SARS-CoV2, the virus responsible for COVID-19 disease. The aim is to establish “a scaled process under current good manufacturing practices (cGMP), capable of supporting…
Charles River: COVID forces closure of donor clinic disrupting HemaCare biz
Charles River says its cell therapy services business HemaCare is susceptible to the impact of COVID-19 and has already had to temporarily close its donor collections clinic. Charles River Laboratories acquired HemaCare for $380 million in December last year, bolstering its cell therapy services business. HemaCare provides human primary cells as well as services used in the discovery, development and commercial production of cell therapies, and provides leukapheresis services for materials used in the manufacture of Novartis’ Kymriah (tisagenlecleucel), Kite’s…
Univercells on balancing its bioprocess tech and CDMO subsidiaries
Univercells has launched a bioprocess technology subsidiary it says can offer a new generation of biomanufacturing platforms that can solve industry’s manufacturing challenges. It has been a busy few months for Belgium-based biologics platform firm Univercells. In February, global investment firm KKR – through its portfolio of life sciences tools companies Gamma Biosciences – committed €50 million ($54 million) into the firm Univercells. Then last month, the firm launched Exothera, a process development and viral vector contract development and manufacturing…
Fujifilm licenses AAV vector tech to speed gene therapy production
CDMO Fujifilm Diosynth Biotechnologies (FDB) says partnering with OXGENE could reduce the lead time of its customers’ gene therapy projects by up to 25%. The technology, licensed from UK-based OXGENE for an undisclosed fee, consists of Helper, Rep/Cap and Gene of Interest plasmids, used in combination with a clonal suspension a HEK293 cell line. The AAV system is expected to reduce the length of the supply chain gene therapy customers, according to contract development and manufacturing organization (CDMO) FDB, with…
MicrofluidX to take on single-use tech with microfluidics for CGTs
MicrofluidX will use fundraising money to advance its cell bioprocessing technology, which uses microfluidics to tackle challenges associated with bioprocessing advanced therapies. The London, UK-based firm has raised £1.4 million ($1.7 million) in seed funding to advance its microfluidics-based technology, which aims to combat challenges associated with the production of cell and gene therapies (CGTs) such as cost of goods, batch variability, and scalability. “Cell and gene therapies have demonstrated significant therapeutic potential. However, these treatments are now at a…