Novo Nordisk will pay Asterias $2 million to lease a facility in Fremont, California for the supply of stem cell-based therapies. An manufacturing tech licensing deal is also an option. Danish drugmaker Novo Nordisk intends to set up manufacturing capabilities for its clinical phase stem cell therapies for type 1 diabetes and other chronic diseases from 2019 onwards after subleasing the plant in California from Asterias Biotherapeutics. The 44,000 square feet current good manufacturing practices (cGMP) facility is used as…

Facilities & Capacity
Novartis buys 9% stake in Chinese Kymriah manufacturing partner
Novartis has paid $40 million for a 9% stake in Cellular Biomedicine Group (CBMG), a Shanghai-based firm which will manufacture CAR-T cell therapy Kymriah for the China market. Under terms of the deal, CBMG will take responsibility for the manufacture of the chimeric antigen receptor (CAR) T-cell therapy Kymriah (tisagenlecleucel) from its facility in Shanghai, China, on behalf of Novartis entity Beijing Novartis Pharma for supply in China. āAligned with our global supply and regulatory strategy, Novartis actively has been…
The POD couple: Pall and G-CON team on modular cleanrooms
Pallās continuous bioprocessing technologies will be incorporated into G-CONās prefabricated āPODā cleanroom units in efforts to address current capacity constraint, CAPEX deferment, and cost predictability. G-CONās PODs are mobile cleanroom units, which include integrated process piping and heating and air conditioning (HVAC) system. A collaboration with bioprocessing vendor Pall Biotech will bring its continuous bioprocessing and viral vector production designs, including automation and utility supplies, to the units. The partnership sets out to solve three needs: contract development and manufacturing…
Emergent extends ABEC disposable deal to equip Maryland plant
Emergent BioSolutions has extended its partnership with vendor ABEC and added a dual purpose single-use system at its site in Baltimore, Maryland. ABEC has shipped a custom designed CSR (Custom Single Run) dual purpose single-use system to Emergentās Bayview facility, intended for large scale microbial fermentation and mammalian cell culture. āA CSR dual purpose system can operate as a 750 L production microbial fermenter or as a 500L seed bioreactor for Emergentās 4,000 L CSR bioreactor,ā ABEC spokesperson Susan Cooper-Curcio…
Hey Praesto: Purolite opens Protein A plant, opens up resin market
Purolite says its 100,000 L facility will give biomanufacturers the āonly credible alternativeā for the supply of agarose Protein A resin. The firm claims its chromatography resins can reduce costs by up to 70% compared to the market leading product. This week, Purolite Life Sciences cut the ribbon at a manufacturing facility in Llantrisant Business Park, Wales capable of producing 100,000L of its Praesto range of agarose Protein A affinity resins. Supported by a Welsh government grant, the facility is…
Three-way collaboration looks to scalable purification for AAV
The UKās Centre for Process Innovation (CPI) will work with CDMO Cobra Biologics and vendor GE Healthcare to reduce the cost of making adeno-associated virus (AAV) vectors. Funded by a Ā£570,000 ($750,000) grant from Innovate UK, the three-way consortium is looking to increase the robustness and reduce costs for the manufacturing of adeno-associated virus (AAV) vectors to feed the growing gene therapy space. The collaboration will see each player contribute its expertise. GE Healthcareās Puridify fibre-based chromatography technology platform will…
Celltrion āwrapping upā 50,000L expansion in Korea; eyes third plant
Celltrion says an additional 50,000 L of bioreactor capacity will come online next year. Meanwhile, the Korean drugmaker is āundecidedā on the location of a third biomanufacturing facility. A capacity expansion at Celltrion is close to completion, doubling the size of the firmās first facility at its site in Songdo, Incheon, South Korea. āCelltrion is currently wrapping up steps to add 50,000 liters of expanded capacity to its first plant based in Incheon,ā spokesperson Heewon Park told BioProcess Insider. Presently,…
AGC adds mammalian capabilities to Japanese network
Demand for mammalian biomanufacturing services has led AGC Bio to invest in its facility in Chiba, Japan. Contract development and manufacturing organization (CDMO) AGC Bio is adding a new facility at its site in Chiba containing single-use bioreactors at the 500 and 2,000 L scale. The plant, expected to be operational by the second half of next year, will produce monoclonal antibodies (MAbs), fusion proteins and other types of therapeutic proteins for its customers. āThe facility is being established for…
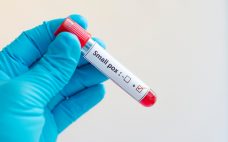
UK orders smallpox vaccine in response to monkeypox occurrences
Bavarian Nordic has supplied volumes of its smallpox vaccine for personnel involved in the treatment of two UK patients diagnosed with monkeypox. At the time of going to press, two patients have been diagnosed with the potentially fatal but rare disease monkeypox in the United Kingdom. Both are believed to have contracted the disease in Nigeria, where there has recently been a monkeypox outbreak. While there is no specific treatment or vaccine for the viral zoonotic disease, Public Health England…
Lonza targets both early and late-stage biotech in $415m Ibex expansion
CDMO Lonza has invested CHF 400 million in a site in Switzerland to expand its Ibex Solutions business, adding end-to-end services for biotech customers. Contract development and manufacturing organization (CDMO) Lonza has launched today Ibex Design and Ibex Develop, offering smaller biopharma companies complete product lifecycle management. The CHF 400 million ($415 million) expansion follows the launch of the Ibex Solutions service in July 2017, which saw the firm look to provide flexible production capacity for biotherapeutics through a biopark…