Orchard Therapeutics says it will balance its existing CMO network with a planned facility in Fremont to create the infrastructure needed to commercialize its gene therapies. Orchard’s pipeline of ex vivo autologous gene therapies include the former GSK hematopoietic stem cell (HSC) gene therapy Strimvelis – approved by the EMA in 2016 – and five clinical stage programs. The firm has been reliant on its network of contract manufacturing organizations (CMOs) to produce these therapies, but late last year it announced it…

Facilities & Capacity
CMOs to continue picking up Big Pharma non-core facilities
Recent facility acquisitions by Lonza and Catalent show Big Pharma is divesting its non-core assets in a trend that is set to continue, according to PharmSource. In the previous couple of months, contract manufacturing organizations (CMOs) Lonza and Catalent have both increased their production networks through facility acquisitions. Lonza’s expansion incorporates a sterile, multi-product drug product facility in Stein, Switzerland, acquired from fellow Swiss firm Novartis. Catalent, two weeks prior, added its first biologics plant outside the US through a…
Bio-Techne: $50m reagent plant will support cell & gene therapies
Plans for a $50 million GMP reagent facility in Minnesota are materializing at Bio-Techne as it looks to support the burgeoning cell & gene therapy sector. In its third quarter FY2019 financial call, life sciences services and consumables firm Bio-Techne spoke of intentions to build a $40 million (€36 million) GMP facility in Minneapolis, Minnesota to produce reagent proteins for use in cell and gene therapy applications. Three months on, the firm has said plans are now laid to invest…
Samsung BioLogics to make third UCB drug substance
CDMO Samsung BioLogics will make a minimum of $70 million worth of three products for UCB from its facilities in South Korea. A spokesperson from Samsung BioLogics told this publication the extended deal with Belgium-headquarters pharma firm UCB means a third drug substance will be made by the contract development and manufacturing organization (CDMO) from its site in Songdo, Incheon. The two companies entered into a master services agreement in July 2018. The latest product to be made by Samsung…
Novo Nordisk to convert Purdue NC plant to make oral diabetes drug
Novo Nordisk will rebuild a facility in Treyburn, North Carolina acquired from Purdue Pharma to support an oral form of its GLP-1 analogue semaglutide. In an email sent to staff and posted on cafepharma.com, Craig Landau – CEO of OxyContin maker Purdue Pharma – said an evaluation of his firm’s manufacturing operations and capabilities has resulted in the sale of its Treyburn solid oral dose plant to Danish drugmaker Novo Nordisk. Financial details of the deal have not been divulged,…
Shingrix a ‘standout’ in GSK’s vaccine strong Q2
GlaxoSmithKline (GSK) has described its shingles vaccine Shingrix as a driving force in its Q2 sales results. The firm is expanding capacity to keep up with demand. For the second quarter 2019, group sales stood at £7.8 billion ($9.8 billion), up 7% year-on-year. GSK saw a 1% decline to £4.3 billion in its pharmaceuticals division but was boosted by strong performances in vaccines and consumer. Management attributed vaccine sales of £1.6 billion – up 23% on the same period last…
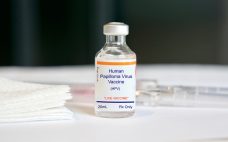
Merck builds up Gardasil network again with $680m NC injection
Merck & Co. will build a 225,000 square-foot facility in Durham to support production of its HPV vaccine Gardasil, creating 400 jobs. Merck will report its second quarter 2019 results on July 30, but for the previous quarter the firm saw a 31% year-on-year increase in sales of its human papillomavirus (HPV) vaccines Gardasil and Gardasil 9, which pulled in sales of $838 million (€752 million). Adam Schechter, Merck’s president of Global Human Health, said last year that his firm…
Lonza building out capacity to prep for ADC boom
CDMO Lonza already supports three of the five commercially available antibody-drug conjugates (ADCs) and is adding two news suites in Switzerland in preparation of future demand. Contract development and manufacturing organization (CDMO) Lonza will expand its bioconjugation facility in Visp, Switzerland in readiness for a new wave of antibody-drug conjugates (ADCs) coming through the clinic. “Many bioconjugates are on expedited programs and the existing expertise at the facility, combined with proximity to clinical and commercial manufacturing of antibody, linkers and…
AveXis: ‘Speedy scale-up driven by Novartis acquisition’
With its own facilities, support from Novartis and now dedicated space at Catalent’s Paragon, AveXis says it has the most gene therapy manufacturing capacity in the industry. AveXis has inked a deal with contract development and manufacturing organization (CDMO) Paragon Gene Therapy to secure dedicated manufacturing space for its one-off spinal muscular atrophy (SMA) treatment Zolgensma (onasemnogene abeparvovec), approved in the US in May. Paragon’s facility in Maryland will also provide process development for clinical supply of other gene therapy…
Gilead CA site to house viral vector plant for Kite
Kite will build a 67,000 square-foot facility within its parent company’s site in Oceanside, California to ensure sustainable viral vector supply for its cell therapy products. The Oceanside site will advance viral vector development and supply for both Yescarta (axicabtagene ciloleucel) and its pipeline therapies, according to Kite spokesperson Shant Salakian. “This new facility in Oceanside, CA will be dedicated to the manufacturing of viral vectors and will help ensure we have the capabilities to support a robust and sustainable…