This webcast features: Dr. Sean Liour, Vice President for Project Management, GenScript ProBio A cell line platform can provide the best solution for the biopharmaceutical target. This webinar explains how to develop a robust stable line platform for CMC projects. GenScript’s biologics platform can provide one-stop service of CMC for the biopharmaceutical target. Watch the recorded webcast now.
Ask the Experts
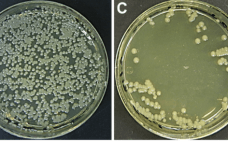
Cryogenic Temperatures and Cell Viability
This webcast features:Â Dr. Peter Kilbride and Dr. Julie Meneghel, Senior Research Scientist and Cryobiologist, GE Healthcare Life Science In this webinar, we will address fundamentals of cryobiology to (1) understand why a cryopreservation protocol is needed, (2) see what happens biologically to cells while cooling, and (3) allow attendees to design an optimized protocol for their cell type and cryopreservation configuration (e.g., sample size). We will cover the importance of applying controlled-rate cooling and how to best tune it to…
Ask the Expert: Accelerating Development and Manufacturing Platforms for Viral Vectors
Bai-wei Gu, Juan Lagos, and Matthew Weaver (heads of cell line development, upstream process development, and downstream process development groups, respectively, at WuXi Advanced Therapies, ATU) joined forces on 29 October 2019 to feature their company’s viral-vector manufacturing capabilities for cell and gene therapies. In addition to adherent platforms for lentivirus (LV) and adenoassociated virus (AAV) vectors, ATU soon will offer suspension-cultured viral vector platforms for them as well as analytical measures that support release testing. Transitioning from adherent to…
Ask the Expert: Best Practices for Aseptic Sampling from Stainless-Steel Equipment
Turn-key single-use aseptic sampling devices (ASDs) have diminished bioprocess contamination risks significantly. But depending on testing, facility, and storage needs, some ASD container types are more effective than others are. Bobbi Allen (technology expert at Sartorius Stedim Biotech North America, SSB) focused her 8 January 2020 “Ask the Expert” presentation on “what, why, when, and where” operators must sample aseptically from stainless-steel tanks. Using data from in-house testing of aseptic sampling containers, Allen offered key considerations for sterility, process monitoring,…
Introducing New Digital Tools to Enhance Raw Material Verification
This webcast features: Antonia Guerra, Global Digital and Data Science Leader, Field & Safety Instruments, Thermo Fisher Scientific™ This webinar presents the new virtual companion to the Thermo Scientific™ TruScan™ RM Handheld Raman Analyzer, the Virtual TruScan™ RM (VTR) App, which creates efficiencies in material identity verification. This first-of-its-kind digital tool puts the TruScan RM’s decision algorithm in the cloud and allows for method validation and spectral re-processing without the need for a physical sample. With the VTR app, wait…
Ask the Expert: Highly Sensitive Host-Cell Protein Analyses Using Novel Chromatography Technology
Geert Van Raemdonck (global field support expert at PharmaFluidics) and Koen Sandra (scientific director of the Research Institution for Chromatography, RIC) teamed up for a 10 October 2019 “Ask the Expert” webinar to introduce micro Pillar Array Column (ÎĽPAC™) technology for liquid chromatography–mass spectrometry (LC–MS) for host-cell protein (HCP) detection. Van Raemdonck explained that ÎĽPAC technology approaches chromatography differently than does packed-bed technology. Microfluidic channels with arrays of free-standing pillars are etched lithographically into a silicon wafer. The resulting permeability…
Ask the Expert: Accelerating Timelines By Integrating Cell-Line Development and Manufacturing
In a 31 October 2019 “Ask the Expert” presentation, Nicole Wakes (group leader of Abzena’s cell-line development team) observed that drug sponsors often outsource their early upstream activities to a few different contract research organizations (CROs). But that strategy can thwart short timelines and introduce regulatory and financial risks. Wakes described Abzena’s upstream approach, illustrating how partnering with a single, multicompetent CRO from cell line construction through manufacture can streamline workflows. Integrating cell line development and manufacturing in this way…
Ask the Expert: Developing Bioprocesses for Clinical Manufacturing Success
Biopharmaceutical companies need to make critical chemistry, manufacturing, and controls (CMC) decisions during clinical development of recombinant protein biologics and advanced therapies. In a 17 December 2019 “Ask the Expert” webinar, Nigel Shipston (director of program design at FUJIFILM Diosynth Biotechnologies, FDB) reviewed key aspects of selecting and working with a contract development and manufacturing organization (CDMO). He also highlighted important factors that should be considered during early stages of process development. Shipston’s Presentation The sheer magnitude of investment required…
Ask the Expert: Cell Culture Media Analysis Using Handheld Raman Analyzers
In biopharmaceutical manufacturing, cell culture media supply critical nutrients and maintain pH and osmolality to optimize protein product yield. Because media composition and condition have a strong effect on final biologic product quality and production, biopharmaceutical companies monitor media for lot-to-lot variability. Stability testing for degradation due to light exposure, temperature changes, or shelf-life/time is possible with rapid spectroscopic methods. In an 8 October 2019 “Ask the Expert” webinar, O. Dean Stuart (product manager at Thermo Fisher Scientific) explained how…
Best Practices for Aseptic Sampling from Stainless Process Equipment
This webcast features: Bobbi Allen, Technical Expert, Aseptic Sampling, Sartorius Stedim Biotech Aseptic sampling from stainless process equipment is a regulatory requirement and can be accomplished in many ways. How samples are removed from the process stream and the containers you collect them into can have an impact not only on the integrity of the process but the accuracy and repeatability of the tests you are performing. Emerging technologies can streamline sample taking, minimize risk to process, and improve the…