As head of bioanalytics at Sanofi, Claire Davies leads a team of >90 people involved in development, qualification, and transfer of methods to internal and external commercial quality control units; characterization, comparability, and developability of proteins and gene therapies; and analytical support for up- and downstream development, production, product release, and stability testing. She has served in a number of roles over 18 years with Sanofi, from chemistry, manufacturing, and controls (CMC) leadership of preclinical to commercial products (leading and…
Upstream Development
A Rapid, Low-Risk Approach Process Transfer of Biologics from Development to Manufacturing Scale
Successful scale-up of cell culture for manufacturing of biopharmaceuticals gives companies time to accelerate clinical development, product commercialization, and market access (1). Scaling a cell culture process in stirred-tank bioreactors ideally includes optimizing that process at laboratory scale and then transferring it through larger pilot-scale and finally to manufacturing-scale bioreactors (2). This is a complex, time-consuming business that can involve process transfer — sometimes to different geographical locations and through many sizes of bioreactors, each of which can operate according…
A Future-Proof Solution for Bioprocess Applications: The New Eppendorf Flexible Bioreactor Control System Evolves with the Changing Needs of Modern Biotechnology
In the biopharmaceutical industry’s quality-by-design (QbD) era, optimizing tools for process monitoring and control has become a major focus of development and manufacturing. This increased attention brings challenges into upstream and production processes, cell-line development, process optimization, and scale-up. Suppliers of equipment and technologies also focus on helping their customers improve development timelines. With that increased attention to speed, they are offering tools such as the Eppendorf SciVario twin bioreactor control system to streamline development and maximize flexibility. BPI spoke…
Improving Bioprocess Expression Systems: A Clean Alternative to CRISPR/Cas9
Chinese hamster ovary (CHO) cells have emerged as a robust platform for bioprocessing serving both early and late-stage biotherapeutic drug supply. However, these cells and other hosts (e.g., HEK293), can be optimized for even greater potential through advanced gene editing. For example, when the endogenous glutamine synthetase (GS) gene is knocked out in CHO cells, a sixfold increase in high-producing cell lines is achieved (1). In another study, CHO with annexin A2 (ANXA2) and cathepsin gene (CTSD) knockouts were introduced…
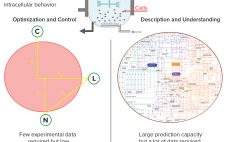
From Big Data to Precise Understanding: The Quest for Meaningful Information
High-throughput technologies have transformed the biotechnology industry. The amount of data they generate is at least a hundred times higher now than it was two decades ago, primarily because of the rise of “-omic” technologies. As in many other industries, the biopharmaceutical sector entered the era of big data the day that high-throughput analytics were routinely implemented in experimental research. Big data refers to “datasets with sizes beyond the ability of commonly used software tools to capture, curate, manage, and…
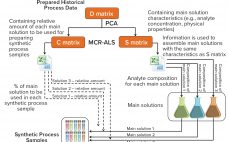
Bioprocess Development and Qualification: PAT-Based Stage 1 and 2 Acceleration Strategies
Well-established process analytical technology (PAT) strategies, such as those based on spectroscopy, bring with them several challenges related to the nature of those tools themselves (1–3). Such tools are multiparametric by design — in the sense that most spectroscopies capture multiple attributes sometimes different in nature (e.g., near-infrared, NIR, captures chemical and physical attributes simultaneously). Often a reference method is required; at other times, indirect calibrations are based on the correlation of one culture attribute with another for which a…
Ask the Expert: Developing Bioprocesses for Clinical Manufacturing Success
Biopharmaceutical companies need to make critical chemistry, manufacturing, and controls (CMC) decisions during clinical development of recombinant protein biologics and advanced therapies. In a 17 December 2019 “Ask the Expert” webinar, Nigel Shipston (director of program design at FUJIFILM Diosynth Biotechnologies, FDB) reviewed key aspects of selecting and working with a contract development and manufacturing organization (CDMO). He also highlighted important factors that should be considered during early stages of process development. Shipston’s Presentation The sheer magnitude of investment required…
Upstream: Make the Right Decisions for Your mAb
Bioprocess decisions made during upstream operations can be difficult to reverse at later, more costly stages of biologic manufacture. They even can require significant backtracking, wasting precious time, labor, and material. Read this Special Report to learn ways to optimize monoclonal antibody bioprocessing upstream. Specifically, you will learn about different tools that small and emerging biotechnology groups can use to ensure robust cell-line selection novel media formulations designed for intensified upstream processing in perfusion modes mixing and delivery solutions that…
eBook: Addressing Production Complexities — Strategies for Working with Difficult and Susceptible Proteins
All proteins are complex — but some are more complex than others, particularly when it comes to recombinant protein expression and production in commercial quantities. What works in a research laboratory to make a milligram of pure protein for study won’t necessarily work on a manufacturing floor to make kilogram batches for drug-product formulation. An increasing number of technological options are available, however, from a simple switch in expression host or adding folding steps in downstream processing to special genetic…
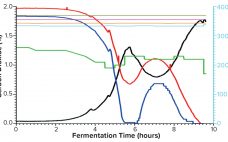
Comparative Study of Single-Use and Reusable Fermentors: Production of Recombinant Proteins Through Bacterial Fermentation
Single-use bioreactors have become widely accepted and well established for cell culture applications in the biopharmaceutical industry for over a decade (1). Abbott Diagnostics has moved into this technology already for commercial production of some biologic molecules. However, single-use systems (SUSs) are rarely available for microbial applications, mostly because of the technical challenge in designing cost-effective SUSs that can meet high oxygen transfer needs and remove excessive heat generated during fermentation. Thus, an important part of our biologics manufacturing —…