The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment will continue to…
QA/QC
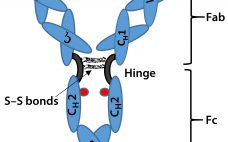
Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 2
Last month, Part 1 of this discussion briefly described the regulatory landscape for developing biosimilar therapeutic monoclonal antibodies (TMAbs). We identified certain specific structural components of TMAb drug substances that warrant particular attention because alterations to them are likely to affect therapeutic safety and effectiveness. Now we conclude by considering whether studies of reference materials can further the development of analytical industry standards to ensure comparability of putative biosimilar TMAbs with innovator TMAbs. We suggest that the time is right…
Mass Spectrometric Conjugate Characterization: Process Qualification of Recombinant Protein–Hapten Conjugation
Conjugated protein biotherapeutics such as PEGylated proteins (with polyethylene glycol), antibody–drug conjugates (ADCs), and protein–haptens often present unique analytical challenges related to characterizing the conjugation aspect of their manufacturing processes. Analytical characterization of this class of proteins requires knowledge of the sites of conjugation, the degree of conjugation, and the drug-to-protein ratio. Here we present case studies in development of reliable methods based on mass spectrometry (MS) to characterize a protein–hapten drug substance during late-phase process validation. This protein is…
Providing Lipids Boosts Protein Productivity: Testing a Feed Supplement with Multiple Cell Clones and Media Formulations
As the biologics (and now biosimilar) markets continue to grow, pressure increases on biomanufacturers to reduce cost of goods sold (CoGS). One way they can reduce cost is by increasing protein productivity in terms of protein titer per volume of culture. Media optimization is a key strategy for increasing protein productivity. In the past few decades, average titers across the industry have increased greatly — from <0.5 g/L in the 1980s to >3 g/L today, and it is not uncommon…
Advanced Protein Engineering Enhances Biopharmaceutical Manufacturing and Analytics
Production of proteins for pharmaceutical use is a complex, multistep process that requires technologies for purifying such molecules from highly complex biological mixtures. It also calls for reliable, cost-effective, high-throughput analytical techniques to determine protein quality and functionality to ensure the safety and efficacy of end-products. Mistakes in product development and manufacturing not only are immensely costly, but they can also put patients at risk. Many well-established processes and analytical tools are available for use in manufacturing antibody drugs (e.g.,…
Special Report: A Strategy for Cost-Effective Capture Using Agarose-Based Protein A Resins
It is well recognized that the cost of Protein A resins is substantial. If a developmental monoclonal antibody (MAb) makes it to marketing approval and manufacturing, the high cost of purification using a Protein A resin is amortized over a large number of purification cycles, and the contribution to cost of goods is reduced to acceptable levels. However, a high percentage of clinical projects will fail, and the Protein A resin will be used only for a small number of…
Progress Toward Commercial Scale and Efficiency in Cell Therapy Bioprocessing
Regenerative medicine includes both cell and gene therapies. Currently 672 regenerative medicine companies operate around the world, and 20 products have been approved by the US Food and Drug Administration (FDA). Of 631 ongoing clinical trials by the end of 2015 (1), over 40% are in oncology, followed in prominence by cardiovascular and infectious diseases. Here I focus on gene and cell therapy bioprocessing in which the final products delivered to patients are cells. Cell therapies are either autologous (derived…
Viral Risk Evaluation of Raw Materials Used in Biopharmaceutical Production
Ensuring a continuous supply of safe medicines to patients is a key objective for both health authorities and the pharmaceutical industry. A critical component to that end is maintaining a reliable supply of qualified raw materials (RMs). Manufacturers must ensure not only the suitability of RMs for their intended use in a manufacturing process, but also their highest attainable safety with regards to viruses and other adventitious agents. The need to apply a risk-based RM control strategy is in line…
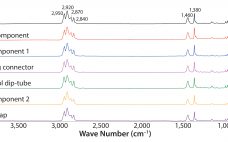
Investigation of Foreign-Particle Contamination: Practical Application of FT-IR, Raman, and SEM-EDS Technologies
The presence of visible foreign particulate matter is considered a critical defect in parenteral products and one of the main reasons they can be recalled (1). Foreign particles present during any stage of manufacturing are considered to be contaminants and can impose a risk to the control of the manufacturing processes (2). For those reasons, particle contamination arising in any manufacturing step initiates a nonconformance or out-of-specification observation. That requires an investigation to identify root cause so as to mitigate…
Science, Risks, and Regulations: Current Perspectives on Host Cell Protein Analysis and Control
State-of-the-art analytics guide process development by providing companies with thorough understanding, effective removal, suitable control, and comparability assessment after process changes of host cell proteins (HCPs) in recombinant biotechnology products. An array of analytical techniques and approaches can be used to establish control strategies for host cell proteins. Techniques used for HCP characterization and comparability include two-dimensional (2D) gel electrophoresis with a range of stains, 2D immunoblotting, 2D high-performance liquid chromatography (HPLC), 2D difference gel electrophoresis (DIGE), and increasingly mass…