The primary goal of container–closure integrity (CCI) is to maintain the sterility and product quality of parenteral biopharmaceuticals throughout their shelf life and use. Guidelines detailing the initial CCI qualification and validation requirements have been defined and can be found in the US Pharmacopeia chapter 1207 (USP<1207>) (1). The guidelines described in USP<1207> can be applied to any common CCI testing (CCIT) method to achieve a method suited for its intended use within a drug product lifecycle. CCI is not…
QA/QC
Setting Up a Rapid Mycoplasma Assay to Support Recombinant Protein Production
Octapharma AB (OAB) in Stockholm, Sweden, is the site for Nuwiq human recombinant factor VIII (FVIII), production. The drug is produced in a human cell line cultured in a perfusion bioreactor using a closed system (to minimize contamination) and proprietary serum-free medium without animal-derived components. In accordance with regulatory guidelines, cell banks and cell cultures used for production of biological products must be free of mycoplasma. Traditional mycoplasma testing is a growth-based method that represents a significant bottleneck in quality…
eBook: Quality By Design for Monoclonal Antibodies — Establishing the Foundations for Process Development, Design Space, and Process Control Strategies
The quality by design (QbD) modernized approach to pharmaceutical development is intended to provide regulatory flexibility, increased development and manufacturing efficiency, and greater room to innovate as well as improve manufacturing processes within defined ranges without obtaining regulatory approval first. QbD is a systematic developmental approach that starts with a clear goal in mind and emphasizes understanding of how variability in both process and materials affects a final product (1). Historically, product quality has been assured either with end-product testing…
Methods on the Move: Addressing Method Transfer Challenges for the Biopharmaceutical Industry
Analytical method transfers are essential components of the current global biotechnology environment. Analytical method transfer can be defined as “a documented process that qualifies a laboratory (the receiving laboratory) to use a validated analytical test procedure that originated in another laboratory (sending laboratory), thus ensuring that the receiving laboratory has the procedural knowledge and ability to perform the transferred analytical procedure as intended” (1). The goal is to ensure that a method continues to perform in the validated state regardless…
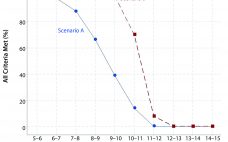
Statistical Assessments of Bioassay Validation Acceptance Criteria
Analytical linearity as well as assessments of precision and accuracy determine the range for a bioassay (1). USP <1033> recommends comparing confidence intervals (CIs) against target validation acceptance criteria in a bioassay validation exercise, but there are no clear guidelines for determining the criteria (2). Here I address several aspects of a bioassay validation, namely • Linearity (coefficient of determination (R2), slope, and intercept parameters) • Accuracy (%relative bias, %RB) • Precision (percent coefficient of variation, %CV) CIs for the…
Ensuring the Integrity of Single-Use Containers: Providing Robustness, Science, and Helium-Based Technology with a Detection Limit of 2 ÎĽm
Identifying the greatest defect size, both for liquid leaks and microbial ingress, is a fundamental step toward protecting the integrity of single-use systems (SUS) under real process conditions. Integrity testing of such systems may become a prerequisite in the future because they are used in the most critical process steps, with detection limits correlating to liquid leaks and microbial ingress. Such testing guarantees the sterility of drug substances and drug products packaged in single-use systems and, therefore, enhance patient safety.…
Antibody Higher Order Structure Stability: Polymorphism Revealed By Protein Conformational Array
For protein therapeutics and other biologics, the importance of the molecule’s structure to its efficacy and safety is well established (1–5). In particular, their tertiary and quaternary structures play very important roles in product quality and have been monitored extensively in comparability studies (6–12). However, because of both the large molecular size and rotational property of amino acid α carbons, a protein can assume an enormous number of different conformations (13). For antibody-based biologics such as monoclonal antibodies (MAbs), fusion…
Viral Vector Particle Integrity and Purity Analyses in Early Process Development
Gene therapy is the transfer of genetic material to a patient’s cells to achieve a therapeutic effect. Therapeutic DNA typically is delivered using a viral vector system, and adenoviruses have been used for this purpose for over 20 years (1–3). Within the past 10 years or so, lentiviruses have shown promise in clinical trials (1–3), and adenoassociated viruses (AAVs) have been used in the first approved gene therapies in the Western world (4). The number of gene therapy applications based…
Development of a Host-Cell Protein Platform Assay for a Chinese Hamster Ovary Cell Line
The Chinese hamster ovary (CHO) cell line is the most prevalent biopharmaceutical expression system and has been proven safe for commercial production of protein therapeutics (1). However, even after multiple purification steps, biopharmaceuticals contain residual host-cell protein (HCP) impurities that pose a potential safety risk to patients (2). Health authorities demand close monitoring of HCP impurities and require sensitive analytical methods with high coverage: the ability to detect a broad range of HCP impurities (3, 4). Polyclonal sandwich immunoassays are…
Regulation, Analytical, and Process Issues with Leachables: Toward Harmonization for Latin America with Europe and North America
The pharmaceutical industry follows strict regulations regarding impurities, including process-related leachates. Plastic manufacturers use hydrophobic, nontoxic additives for manufacturing containers for use in the pharmaceutical and food industries. However, some issues about dealing with such impurities are not yet resolved. In developing countries, regulators are working on guidelines to help local companies ensure characterization of impurities. In this exclusive editorial eBook, authors from Mexico describe some issues related to plastic leachables in the context of ongoing efforts to harmonize regulations…