The product development team at a gene therapy contract development and manufacturing organization (CDMO) was working on a high-priority drug-substance project for a key client. The material was crucial to that client’s early stage clinical trial, with an immediate value over US$500,000 to both the client and the CDMO. Unfortunately, the bioreactor used in the upstream process — a transfection unit operation for an adenoassociated virus (AAV) vector — had developed an intermittent problem that could force it to shut…
QA/QC
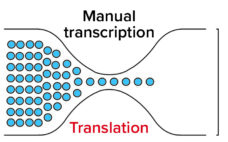
Smart, Real-Time Quality Insights Boost Life Sciences Manufacturing
The COVID-19 pandemic has shone a light on restrictive business processes, information silos, and poor supply-chain visibility in many sectors. In biopharmaceutical manufacturing, for example, difficulties associated with product-quality management have been exposed and starkly felt. However, public healthcare measures over the past 18 months have put physical distance between team members, thereby hampering the usual form-filling, manual sign-offs and spreadsheet-based recordkeeping associated with monitoring traditional manufacturing processes. In some cases, a lack of formal face-to-face discussions in the workplace…
Mycotoxin Risk Determination: Measuring the Potential for Patient Exposure with Antithrombin Alfa Sourced from Transgenic Goat Milk
Antithrombin alfa is a recombinant human antithrombin developed as an anticoagulant treatment for people with hereditary antithrombin deficiency who are undergoing surgical or childbirth procedures (1). Marketed under the ATryn brand name by LFB SA (Les Ulis, France), antithrombin alfa was approved for use in adults by the US Food and Drug Administration (FDA) in February 2009 (2). Antithrombin alfa is expressed in the milk of transgenic goats and purified through a multistep downstream process encompassing both filtration and chromatography.…
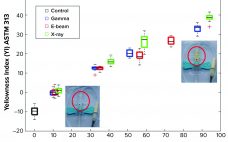
Supplementing Gamma Sterilization with X-Ray and E-Beam Technologies: An International Industry and Academia Collaboration
Ionizing-technology–based industries are growing rapidly around the world. The expansion is driven mostly by the technology’s myriad applications, including polymer crosslinking, medical device sterilization, food pasteurization, and phytosanitary treatment. Ionization also is used in the manufacture of some healthcare products such as medical devices and biopharmaceuticals. Industrial sterilization methods render single-use products and manufacturing components safe and ready for their intended use. ISO11137-1 describes the validation and routine control of a sterilization process for medical devices and mentions the three…
Posttranslational Modifications and Their Control in CHO Culture
The Chinese hamster ovary (CHO) cell line was first established by Theodore Puck in the 1950s and was used mainly for cytogenetics studies (1). Since the 1990s, CHO cells have increased in popularity as expression host cells because they can be adapted easily into suspension culture and genetically modified. The CHO cell line also has a human-like glycosylation profile (2–4). Therapeutic proteins undergo different posttranslational modifications (PTMs) during manufacturing. Some modifications can lead to undesired effects such as decreased stability,…
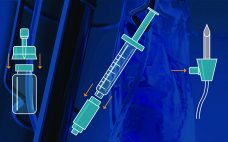
Closed-System Transfer Devices: Collaboration Provides Tools to Guide Compatibility and Stability Testing Strategy
Ever since the first biopharmaceutical product (biologic) was approved in the 1980s, companies have developed protocols and tests to ensure that such products are safe and effective. Biologics are very different from traditional small-molecule drugs, with unique risks inherent to their manufacturing processes. Biopharmaceutical formulations often present as complex mixtures that can be sensitive to heat, light, and many other factors, all of which must be monitored and assessed. However, until recently, developers worked mostly independently, with only their own…
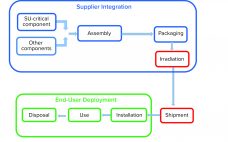
Integrity of Single-Use Systems: Practical Applications and Deployment
Single-use (SU) technology plays an important role in modern vaccine and biologics manufacturing. System integrity, managed by critical process controls, ensures sterility and is a prerequisite for successful leak-free processing. Nonintegral systems cause loss of product, quality, and time; increase costs through investigations; and lead to potential safety problems. The BioProcess Systems Alliance (BPSA) issued a white paper in 2017, Design, Control, and Monitoring of Single-Use Systems for Integrity Assurance (1), that describes in detail the strategies for design and…
Spontaneous Infection: Did You Leave the Back Door Open to Your Cultivation Suite?
Manufacture of biopharmaceuticals using mammalian cells inherently incurs a risk of viral contamination during cell cultivation. If introduced, viruses can infect and replicate in cells used to produce a therapeutic protein or vaccine. The consequences of such contaminations can be dramatic. Not only can a company lose contaminated batches, but it also faces potentially extensive root-cause investigations, facility cleanup efforts, and introduction of preventive measures. Until contamination issues are resolved adequately, production should not be resumed, and facility downtime brings…
Ask the Expert: Leveraging Quality Management Systems to Achieve Competitive Advantages
During a May 2021 “Ask the Expert” presentation, Jigisha Patel (vice president of global regulatory compliance and technical services at Spectrum Chemical Manufacturing Corporation) emphasized the need to minimize supply-chain contingencies that lead to variability across raw materials used in biopharmaceutical manufacturing. Deviations in raw-material specifications can jeopardize good manufacturing practice (GMP) compliance and reduce drug-product efficacy and stability. Patel explained how her company’s quality management system ensures that bioprocess materials will meet specifications and process needs. Patel’s Presentation Patel…
eBook: Process-Related Impurities — Emerging Strategies for Detection, Identification, and Management of Host-Cell Proteins
Host-cell proteins (HCPs) represent a major class of process-related impurities (PRIs) that are generated during biopharmaceutical manufacture. Although the vast majority of such proteins are removed from a drug substance during downstream purification, residual HCPs can remain in a finished drug product. Even in minimal concentrations, copurifying HCPs can pose safety risks and compromise protein-product yield, efficacy, and stability. Thus, regulatory agencies consider the presence of HCPs to be a critical quality attribute (CQA). Sufficient clearance of these impurities helps…