Bioassay development is a complex process that must be undertaken with great rigor and attention to detail. Potency testing experts use a range of methods including cell-based and binding assays. Consistency and reliability of results over time are paramount. Well-developed and -characterized methods are the end result of much phase-appropriate development work that goes on in parallel with bioprocess and biotherapeutic product development. This eBook begins with BPI senior technical editor Cheryl Scott’s report from the Biopharmaceutical Emerging Best Practices…
Product Characterization
eBook: Potency Bioassays — Development, Trending,
AAV Downstream Process and Product Characterization: Integrating Advanced Purification and Analytical Tools into the Workflow
The optimization of the downstream process for Adeno-associated virus (AAV) production with consistent quality depends on the ability to characterize critical quality attributes affecting potency, purity and safety of the final product. As the gene therapy field continues to push products through the clinical pipeline, an increasing need for efficient purification and analytical tools has become evident. In addition, the regulatory space has expanded in parallel to the use of AAV, driving the demand for simple and efficient assays to…
Analysis of Trace-Level, High-Risk HCPs: Proteomics Advances for Preventing Degradation of Polysorbates in Biotherapeutic Formulations
Polysorbate-80 (PS-80) and polysorbate-20 (PS-20) are used widely in formulation of biotherapeutic products for preventing surface adsorption and as stabilizers against protein aggregation (1). Degradation of polysorbates can cause turbidity and potential formation of subvisible particles mainly consisting of poorly soluble hydrophobic free fatty acids (1). Polysorbate degradation is an industry-wide challenge both in biotherapeutics processing and formulation development. The risk of such degradation increases with higher cell densities and greater expression titers in bioprocessing, as well as with higher…
Ask the Expert: Critical Steps in Potency Assay Development
Biologics undergo extensive characterization to demonstrate their safety, purity, and efficacy. Jennifer Lawson (product manager for cell line, media, and testing solutions at Sartorius) highlighted the role of potency assays in that process. Because they reflect the complexity of biological systems, scientists must develop robust assays that will provide sufficient data for good-practice (GxP) applications. Lawson pointed out milestones in the bioassay life cycle and explored ways to help ensure method suitability. Lawson’s Presentation Development Criteria: Potency assay development requires…
eBook: Product Variants in Bioprocesses
Product variants are contaminants because they bear properties that are different from those of desired biological products with respect to activity, efficacy, and safety. Thus, such variants can compromise product quality and consistency. In this eBook, Yuval Shimoni explores different types of variants — including primary-sequence variants, undesirable posttranslational modifications, aggregates, and degraded proteins — and explains how their presence can diminish the performance and quality of drug substances and products. He also discusses how product variants form at different…
Analytical Methods for Cell Therapies: Method Development and Validation Challenges
Advanced-therapy medicinal product (ATMP) characterization and analysis play important roles in providing chemistry, manufacturing, and controls (CMC) information for regulatory applications as well as in supporting product-release and stability studies. Each type of advanced therapy presents different analytical development challenges, so each requires specific characterization, potency, purity and identity assays. Variability in cells and among patients, multiple and complex mechanisms of action (MoAs), a general lack of readily available reference materials, and complicated analytical methods and instruments underlie the major…
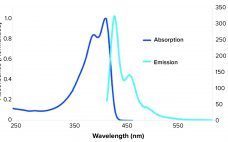
Expanding the Flow Cytometry Toolkit with Next-Generation Polymer Dyes
Since the emergence of SARS-CoV-2, scientists have discovered more about the role of our immune systems and cytokine-associated processes responsible for systemic immune reactions that are typical in patients with COVID-19 (1). Flow cytometry is used to understand those processes at a single-cell level. It is the standard method used in immunology to characterize multiple phenotypic and functional parameters of single cells, including cytokine analysis. Despite advances in flow cytometry instrumentation, reagents, and analytical software, several challenges remain. Conjugated antibodies…
SARS-CoV-2 Hyper-Immunoglobulin: Purification and Characterization from Human Convalescent Plasma
The novel severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) emerged as a major pandemic coronavirus disease in 2019 (COVID-19) and since then has killed many people and paralyzed the global economy (1, 2). With specific antiviral therapeutic agents or antibodies yet to be approved, other antivirals and novel vaccine strategies have been essential to containing the virus and disease transmission. Passive antibody therapy can be used to limit the scope of epidemics by providing patients with antibodies that recognize…
Improving Cell Manufacturing Outcomes Using In-Line Biomarker Monitoring
Cell-based advanced therapies are changing modern medicine dramatically. Immunotherapies such as chimeric antigen receptor (CAR) T-cell therapies are treating different forms of cancer. Gene therapies are reversing the course of inherited diseases, and tissue-engineered medical products are restoring, maintaining, and replacing damaged organs (1–4). The development of new advanced therapies is booming. As of January 2020, the US Food and Drug Administration (FDA) has reported more than 900 investigational new drug (IND) applications for cell and gene therapy products. However,…
Ask the Expert: New and Improved Analytical Methods for Traditional and Unique Modalities
On 10 December 2020, BPI presented an “Ask the Expert” webinar with Jason Sterling, PhD (principal scientist and project director in analytical and formulation resources), and John Rockwell (group leader) of Catalent Pharma Solutions. Biophysical characterization is critical to understanding the makeup and behaviors of biologic therapies and vaccines both early in development and throughout scale-up for manufacturing. As biologics become more complex in structure and as scientists improve their understanding of the effects of structure on stability, efficacy, and…