The market for biopharmaceutical products remains highly attractive to small biotechnology companies and big pharmaceutical corporations alike (1). Most leading market products are made using recombinant technology (2). Pressures are continually increasing on process development groups to reduce development costs and timelines for taking new clinical products forward from product research bench scale into initial clinical evaluation studies. For many years a recognized critical bottleneck in development of products from mammalian cell lines was selection and isolation of stable, high-producing…
Analytical
Trends in Setting Single-Use Technology Standards
The biopharmaceutical industry now incorporates single-use (SU) technology and systems in most production processes based on cell culture (1, 2). Implementation of such technologies has led to the availability of prepackaged and sterilized systems complete and ready for use with preinstalled mixers and monitoring probes. From upstream process- material preparation through final-product formulation, biopharmaceutical sponsors are increasingly presented with numerous SU solutions that support all major production platforms (3–5). The number of SU materials and suppliers in biopharmaceutical manufacturing has…
Understanding and Controlling Sources of Process Variation: Risks to Achieving Product Critical Quality Attributes
Biopharmaceuticals include recombinant proteins, vaccines, gene therapies, and drug products derived from stem cell technology. One key characteristic of shared by all biologics is that they tend to be extremely large molecules with complex three-dimensional structures, critical to their functionality. For example, monoclonal antibodies (MAbs) are composed of more than one thousand times larger than a molecule of aspirin (one analogy compares the complexity of a MAb to that of an F16 jet, and the complexity of aspirin to that…
Upstream Efficiencies, Economic Forces, and Changing Technologies Complicate Separation and Purification
When it comes to biotherapeutics manufacturing, downstream processing groups tend to get “dumped on.” Advances in cell lines, bioreactors, and culture media formulations have increased production output, providing both higher expression titers and greater volumes, but the filters and chromatography columns on the downstream side haven’t kept pace. These century-old technologies haven’t evolved as much and are reaching their limits. Regulatory agencies have contributed to innovation stagnation because they are cautious about manufacturing process changes for fear of undermining quality…
Evolving Clarification Strategies to Meet New Challenges
Increasingly efficient bioreactors allow biopharmaceutical manufacturers to achieve higher cell densities. That improved upstream efficiency has led to new purification challenges resulting from high product and contaminant concentrations as well as complex components. Therefore, harvest and clarification techniques are evolving to incorporate feed pretreatment, flocculation, and different filtration technologies such as normal-flow, tangential-flow, and depth filtration. The objective is to increase process capacities and filtrate quality, ultimately reducing biomanufacturing costs. New strategies for clarification of recombinant proteins (in particular, monoclonal…
Immunoglobulin Fc-Fusion Proteins Part 1: Their Design and Manufacture
Over the past three decades, 45 monoclonal antibody (MAbs) and MAb-derivative products have been approved for therapeutic use in the United States (Table 1). One class of antibody derivatives is growing in importance: Fc-fusion proteins. Many biologically active proteins, including receptor ECDs (see “Abbreviations” box), cytokines, enzymes, and bioactive peptides have very short serum half lives because rapid renal clearance limits their exposure in target tissue (and, consequently, their pharmacological effect). The primary reason for fusing a biologically active protein…
Replacing Reverse-Phase Chromatography for Mass Spectrometry: Is Salt-Free Size-Exclusion Chromatography Ready?
Protein mass is often determined using ultraperformance liquid chromatography (UPLC) coupled with electrospray-ionization mass spectrometry (UPLC/ ESI MS or simply LC-MS). A UPLC system equipped with an ultraviolet (UV) detector serves as an assisting vehicle to deliver purified and separated protein molecules to the mass analyzer. Reserved-phase chromatography (RPC) is the most common chemistry chosen to serve this purpose. For sample purification, not only does RP-UPLC use salt-free mobile phases that are amenable to MS, but it also can efficiently…
Cost Estimation for Protein A Chromatography: An In Silico Approach to MAb Purification Strategy
Monoclonal antibody (MAb) production has adopted an accepted technology platform for downstream processing (1). The need for more economic processes has been addressed by increasing MAb titers in fermentation and aiming toward greater bioreactor volumes to increase productivity. Consequently, cost pressures are now passed on to downstream process groups. Membrane and chromatography resin savings are more important for MAb processes than ever before, with highly productive cell cultures generating large volumes of process fluid to purify (2). Traditionally, protein A…
Preuse, Poststerilization Filter Integrity Testing for Single-Use and Stainless-Steel Installations
According to current European Union good manufacturing practice (EU GMP), integrity testing of sterilizing-grade product filters should be performed preuse poststerilization (PUPSIT) and immediately after use. In addition, PDA’s Technical Report 26 states that preuse integrity tests are preferably performed after filter sterilization. Performing an integrity test of an already sterilized product filter in-line requires wetting the filter while maintaining the downstream side sterile. The test gas must also be evacuted on the downstream side throughout testing maintaining sterility. The…
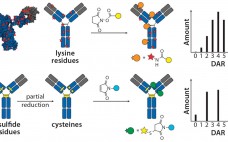
The Next Step in Homogenous Bioconjugate Development: Optimizing Payload Placement and Conjugate Composition
[Audio Recording] Bringing a new biologic drug to market is a long and expensive process, with research and development (R&D) cycles that can span up to 15 years and may cost over a billion dollars. Biologic drug development also involves significantly more complex manufacturing and CMC components than does development of small molecules. Nonetheless, the pharmaceutical industry is increasingly shifting its R&D efforts to focus on biologic drugs. According to a recent report from Tufts Center for Study of Drug…