During the manufacturing of monoclonal antibody (MAb) products, many process reagents are used for cell culture and MAb purification to facilitate and control process performance. Process reagents are considered to be process-related impurities, so demonstration of their clearance is required for the chemistry, manufacturing, and controls (CMC) information submission of an investigational new drug (IND) application (1, 2). These reagents may be classified into two categories: generally recognized as safe (GRAS) reagents and potential safety concern (PSC) reagents (3). GRAS…
Analytical
Optimization and Scale-Up of HCIC-Based MAb Purification Processes, Part 1
Monoclonal antibodies (MAbs) serve important medical needs in cancer treatment as well as that of autoimmune and infectious diseases (1). Antibodies are also widely used in clinical diagnostic assays. They can be coated on solid surfaces to bind specific analytes, conjugated to reporter molecules (either as whole antibodies or fragments) for analyte detection, used in sensitivity panels for lot-release testing, and supplied as positive controls in diagnostic kits (2). Our study evaluates the use of hydrophobic charge-induction chromatography (HCIC) for…
Bioreactor Design for Adherent Cell Culture — The Bolt-On Bioreactor Project, Part 3: Containment, Sterility
The Bolt-on Bioreactor (BoB) project is an independent initiative aimed at developing and commercializing a bioreactor for the automated and efficient culture of adherent cells, especially for application in the production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must answer four major challenges. Addressed in the first article of this series (2), the first challenge has to do…
Foundation Elements for Cell Therapy Smart Scaling
Cell therapy is the injection of cellular material into patients. The injected cell-therapy product (CTP) usually consists of intact living cells. In recent years, cell therapies have evolved and matured, moving from academia to industry. That maturation is reflected in the number of open clinical trials that include the term cell therapy in their descriptions: To date, there are more than 8,700 open trials listed on the US National Institutes of Health’s online database (clinicaltrials.gov), most of which are in…
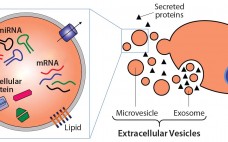
Extracellular Vesicles Commercial Potential As Byproducts of Cell Manufacturing for Research and Therapeutic Use
Extracellular vesicles (EVs) are emerging as a potential alternative to some stem-cell–derived therapeutics (1, 2). Sometimes called exosomes, they are small, secreted vesicles that can possess similar therapeutic mechanisms to whole cells, possibly representing the active pharmaceutical ingredient. In the past 15 years, academic and industry interest in EVs has exponentially increased as mounting evidence demonstrates their role in physiology and pathology as well as their therapeutic potential. In light of growing efforts in using EVs for research and therapy,…
The Potential Application of Real‑Time Release Testing for the Biomanufacture of Autologous Cell‑Based Immunotherapies
Cell-based immunotherapies (iTx) are emerging as a truly transformative therapeutic modality that is both complementary and convergent with existing regenerative medicine approaches, including gene therapy, cell therapy, and tissue engineering (Figure 1). Critically, iTx offer step-change improvements in efficacy compared with current standards of care (1) for a range of clinical indications and unmet therapeutic needs — particularly oncology. The clear efficacy of iTx is in contrast with some previous regenerative medicine approaches, including early mesenchymal stem cell (MSC) therapies…
Hamster Phospholipase B-Like 2 (PLBL2): A Host-Cell Protein Impurity in Therapeutic Monoclonal Antibodies Derived from Chinese Hamster Ovary Cells
All recombinant protein biotherapeutics must be tested for the presence of residual host-cell protein (HCP) impurities (1–3). The most common analytical method for doing so is a polyclonal sandwich immunoassay. Polyclonal anti-HCP antibodies are selected to recognize the broadest population of HCPs possible. The immunogen and analytical standard are produced from a blank-run fermentation that mimics the production run but lacks the specific biotherapeutic protein. Because of the large number of impurities present in harvested cell-culture fluid (HCCF) that might…
Rapid Development and Scale-Up of Biosimilar Trastuzumab: A Case Study of Integrated Cell Line and Process Development
Compared with that for new drugs, biosimilar development faces significantly condensed timelines from cell line to first-in-human (FIH) trials. A biosimilar development program needs to accelerate quickly toward preclinical and phase 1 studies; phase 2 studies typically are not required because dose response and other patient-treatment concepts are already established by the original, comparator medicine. Phase 3 studies typically are limited to fewer patients, which ultimately shortens overall timelines and costs. The key challenge remains: demonstrating comparability and high similarity…
Bioreactor Design for Adherent Cell Culture: The Bolt-On Bioreactor Project, Part 2 — Process Automation
The Bolt-on Bioreactor (BoB) project is an independent initiative to develop and commercialize a bioreactor for automated and efficient culture of adherent cells, especially in production of therapeutic cells and other biopharmaceuticals (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. Addressed in the first installment of this series (2), the first challenge concerns volumetric productivity. The second challenge is…
Building a Robust Biological Assay for Potency Measurement
Potency is a critical quality attribute of a biological product and is often determined by a biological assay (also called bioassay or biopotency assay). Specifically, potency is the biological activity or capacity of a product directly linked to its clinical efficacy. Potency tests are performed as part of product release, comparability studies, and stability testing. Nonbiological methods — which measure a product’s molecular or biochemical characteristics (e.g., ligand-binding assay) — have gained interest as replacements for often troublesome bioassays. Even…