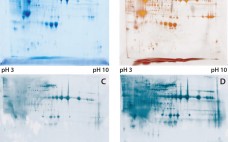
Host-cell proteins (HCPs) constitute an inevitable impurity of biopharmaceutical products originating from recombinant-cell culture. HCPs are a heterogeneous mix of different proteins, their specific characteristics depending on the kind of organism used as an expression platform, on the “destination” of the expressed recombinant product (extra- or intracellular), and on the corresponding purification approach (1–3). Contamination of a final drug substance with residual HCPs could lead to immunogenic reactions in some patients who receive the drug product (DP). So a reliable…
Analytical
The Case for a Standardized Assay to Test Suitability of Single-Use Systems in Cell Culture Applications
Increased commercial use of single-use systems (SUS) for large-scale biopharmaceutical production creates the need for consensus on industry best practices and standards for materials in SUS components. End users and suppliers are beginning to develop a shared vision of industry needs in such areas (1, 2). For example, highly visible efforts to harmonize extractables testing include contributions from groups such as the BioPhorum Operations Group (BPOG), Bio-Process Systems Alliance (BPSA), Parenteral Drug Association (PDA), ASTM, and ISPE. In addition to…
Development, Qualification, and Application of a Bioreactor Scale-Down Process: Modeling Large-Scale Microcarrier Perfusion Cell Culture
Qualified scale-down models of large-scale cell culture processes are essential to conducting studies for applications such as investigating manufacturing deviations, enhancing process understanding, and improving process robustness. For example, scale-down models can be used for raw material investigations as well as evaluation and qualification of new good manufacturing practice (GMP) cell banks for manufacturing implementation. Process characterization studies are performed also with qualified scale-down models to improve process consistency (1, 2). Often it is impractical to conduct investigational studies at…
Heading for a CHO Revolution: The Need for Cell Line Engineering to Improve Manufacturing Cell Lines
The first recombinant protein licensed for use by the United States Food and Drug Administration (US FDA) was human insulin in 1982 (1). That approval was followed in 1987 by the development of tissue plasminogen activator (tPA), the first complex glycosylated protein generated in mammalian cells to be licensed for therapeutic use. Since then, this area of biology has rapidly expanded in clinics: The FDA approved an average of 15 new biological entities every year between 2006 and 2011 (2).…
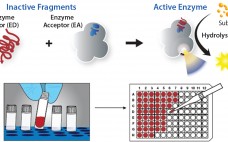
Accelerating Biologic and Biosimilar Drug Development: Ready-to-Use, Cell-Based Assays for Potency and Lot-Release Testing
With the drug industry’s expanding emphasis on biologics, the need for robust cell-based assays has grown at all stages of development. Requirements for efficacy, quality, and potency testing often demand a complex set of bioassays and/or cell-based assays for new therapeutics or biosimilars. Developers of the latter have found this need for cell-based assays to be particularly challenging. Commercially available, ready-to-use cell-based assays provide a robust functional response from specific therapeutic targets. They can significantly shorten assay development time while…
Primary and Higher-Order Structural Characterization Strategy for Biosimilarity Assessment
The development pathway of a biosimilar is unlike that of a novel biotherapeutic. Many regulatory authorities reference a stepwise approach to establishing biosimilarity. Analytical requirements are greatly increased before a product enters clinical testing. Enhanced analytical efforts entail physical, chemical, and biological characterization of a biosimilar product compared with an originator reference product. Strategies at this early stage must include intensive characterization of multiple batches to determine variability of quality attributes. Here I address some issues involved in meeting regulatory…
Outsourcing Stability Testing: Discussions with Contract Laboratories
Stability testing is required for all biopharmaceutical drug products to detect all changes in identity, purity, and potency as a result of a number of environmental and processing factors. Whether testing is conducted in-house or through contact laboratories, it involves the development and performance of comprehensive and specific stability protocols for all stages of a product’s life cycle (1). Testing product stability in-house requires signficant time and resources, and carries challenges associated with commercialization market, time, and capacity. Market: The…
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, Biosimilar Products: Scientific Principles, Challenges, and Opportunities
The Chemistry, Manufacturing, and Controls (CMC) Strategy Forum held on 22 January 2012 in San Francisco, CA, focused on selected scientific and regulatory aspects in the development of biosimilar products. Such products are an increasingly important area of interest for both the biopharmaceutical industry and its regulatory agencies. Biosimilars are highly complex, so scientists have been unable to demonstrate identity to a level typically possible for small molecules. Consequently, specific scientific and regulatory approaches are required to ensure the high…
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, The Role of Higher-Order Structure in Defining Biopharmaceutical Quality
Cosponsored by CASSS (an International Separation Science Society) and the US FDA, the 17th CMC Strategy Forum was designed to explore the relationships between higher-order molecular structure and quality of therapeutic proteins and peptides, vaccines, and blood-derived products. Understanding those relationships is important to defining and controlling the critical quality attributes (CQAs) of biopharmaceutical products. The forum program highlighted the current state of the art for analytical tools used to monitor higher-order structure. Case studies demonstrating the effects of changes…
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, Demonstrating Comparability for Well-Characterized Biotechnology Products: Early Phase, Late Phase, and Postapproval
Challenges and approaches in demonstrating comparability of a well-characterized biotechnology product after manufacturing changes can be as varied and complex as the products themselves. Participants at the January 2005 CMC Strategy Forum sought to discuss and agree on common implementation strategies for different manufacturing change scenarios (1). Development of flexible, comprehensive approaches in strategy development addressed evaluation of critical product characteristics, appropriate process steps to test, numbers of lots and levels of testing required, and assessment of product comparability. The…