Over the past decade, the application of chemical optical sensors for bioprocess monitoring has gradually taken roots. Constant further development of this measurement technology and the possibility to manufacture such sensors in various designs (even for single-use applications) have led to new state-of-the-art devices for the biotechnology sector. Chemical optical sensors enable in situ, real-time monitoring of important culture parameters without sampling and therefore without disturbing a culture. Implementing this technology can decrease workloads and deepen knowledge about bioprocesses. In…
Analytical
Evaluating New Film for Single-Use Bags: Growth Performance Studies with Animal and Human Cells
In biopharmaceutical development and manufacturing processes, single-use technology has become widely accepted (1). Storage and cultivation bags are particularly common. They are fabricated from plastics consisting of multilayer films and are typically provided gamma-sterilized by suppliers (2). The bags offer several advantages such as savings in time and cost. Lowered contamination risk results from reduced cleaning and sterilization demands. However, some adverse effects of polymer films on cell growth and metabolism have been reported, both for storage and cultivation bags…
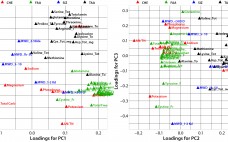
Multivariate Analysis of Biological Additives for Growth Media and Feeds
Biological additives such as yeast extracts and peptones are commonly used in growth-media formulations for biopharmaceutical manufacturing. In spite of drivers encouraging companies to reduce variability in mammalian cell culture processes by using chemically defined media, many microbial and mammalian processes continue to use biological additives in their growth-medium formulations and/or feeds. According to Sheffield Bioscience (Kerry, Inc.), at least six of the top 10 licensed mammalian-cell– derived biotherapeutic products are manufactured using biological additives (1). During process development, it…
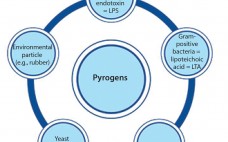
Detecting the Broad Spectrum of Pyrogens with the Human Whole-Blood Monocyte Activation Test
In the early 20th century, some patients injected with the drug Salvarsan experienced febrile reactions due to contamination of the drug’s distilled water. That incident (involving the first effective treatment for syphilis) prompted not only the widespread use of injectable drugs, but also the need for pyrogen control. Pyrogens constitute a heterogeneous group of microbial and nonmicrobial substances that include those derived from Gram-negative and Gram-positive bacteria such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA), respectively, as well as particles…
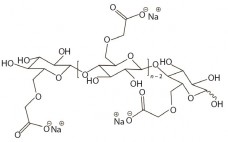
Factors Affecting Sterile Filtration of Sodium-Carboxymethylcellulose–Based Solutions
Carboxymethylcellulose sodium (CMC), is widely used as an excipient in oral, topical, and parenteral pharmaceutical formulations. It increases viscosity (1–3), serves as a suspension aid (4), and stabilizes emulsions (5). More recently, applications for CMC in formulations that facilitate improved delivery of cytotoxic drugs and biologics have been evaluated (6, 7). CMC is manufactured in a broad range of viscosities, with grades typically classified as low, medium, or high viscosity. CMC grades can be divided further based on their degree…
Defining Your Product Profile and Maintaining Control Over It | A Look Back with Emily Shacter
This is a transcript from a Q&A interview with Emily Shacter, PhD, Consultant, ThinkFDA LLC (former FDA Scientist and Regulator). We will be talking today about the CMC Forum that was published back in 2005. We are revisiting it in the magazine to specifically update our understanding of how to maintain process control; understanding your process. In general, how do you feel the discussions in the four-part paper from 2005 has held up after 10 years? Emily: I think they…
Bridging Analytical Methods for Release and Stability Testing: Technical, Quality and Regulatory Considerations
To monitor the control and consistency of products derived from biological systems, a broad array of analytical methods are used for biopharmaceutical release and stability testing. These methods include both classical and state-of-the-art technologies as well as new technologies as they emerge over time.During the life cycle of a product, several reasons can arise for making changes in existing analytical methods: e.g., improved sensitivity, specificity, or accuracy; increased operational robustness; streamlined workflows; shortened testing times; and lowered cost of testing.…
Process- and Product-Relate Impurities: Part 1 – Process-Related Impurities An Overview
Introduction by Cheryl Scott The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated…
High-Throughput Methods Evaluation: Impurities Determination During Upstream and Downstream In-Process Development
Getting biologic drugs through development and into clinical proof-of-concept studies quickly and efficiently is critical for success in the biopharmaceutical industry. Implementing high-throughput approaches to both upstream and downstream process development is increasingly helping companies stay competitive. Innovative and highthroughput analytical technologies are needed to support rapid process development. The study reported herein focuses on innovative immunoassay platforms for impurity-removal monitoring of both host-cell proteins (HCPs) and leached protein A. HCPs come from host cells during cell culture production. Their…
Using Glycosidases to Remove, Trim, or Modify Glycans on Therapeutic Proteins
One of the most common posttranslational modifications of eukaryotic proteins is glycosylation. Glycosylation of proteins can affect many biological activities. For therapeutic glycoproteins, it can modify biological activity, targeting, trafficking, serum half life, clearance, and recognition by receptors (1, 2). For such reasons, biomanufacturers must monitor and characterize the glycosylation patterns of their recombinant therapeutic proteins (3, 4). Therapeutic proteins have two main types of glycosylation: N-linked glycans and O-linked glycans (5). Attachment of an N-glycan starts in the endoplasmic…