The dynamics of the biopharmaceutical industry to get innovative products to the client has evolved over the years. Studies have shown that by 2021, biologics and biosimilar products are projected to have higher growth than other pharmaceutical products. Following the industry trend, Avid Bioservices as a Contract Development Manufacturing Organization (CDMO) has helped numerous clients complete their process validations campaigns. Between 2016 to 2019, Avid has successfully completed six. This custom report will share some key factors to consider for…
Downstream Validation
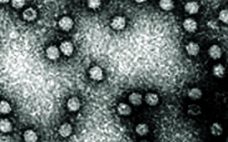
Modeling Virus Clearance: Use of a Noninfectious Surrogate of Mouse Minute Virus As a Tool for Evaluating an Anion-Exchange Chromatography Method
Viral safety is a critical focus during biopharmaceutical manufacturing (1–5). Although well-characterized mammalian cells such as the Chinese hamster ovary (CHO) line have been used for decades, both endogenous expression of retroviral-like particles and exogenous contamination events from viruses warrant continued vigilance (6, 7). International regulatory agencies require biomanufacturers to validate the “viral clearance” efficacy of their downstream manufacturing process steps before resulting products can be awarded clinical trial or commercial approval (8–10). Currently, viral clearance testing is based on…
Emerging Tools for Exosome Purification and In-Process Monitoring
This eBook introduces new analytical approaches that enable in-line chromatographic detection of exosomes. One approach can discriminate extracellular vesicles from nonvesicle contaminants, and one potentially can discriminate exosomes from other vesicles. Examples illustrate how they enable development of more effective and better documented purification methods. The special qualifications of monolithic chromatography media for exosome purification are discussed. New process tools designed to accommodate some of the special challenges of exosome purification are introduced. Exosomes represent one of several species of…
Points to Consider in Quality Control Method Validation and Transfer
The concept of an analytical lifecycle has been well received in the biopharmaceutical industry. In 2016, the US Pharmacopeia (USP) advocated for lifecycle management of analytical procedures (1) and defined its three stages: method design development and understanding, qualification of the method procedure, and procedure performance verification. The US Food and Drug Administration (FDA) has published guidance on process validation with a similar division into three stages: process design, process performance qualification, and process performance verification (2). For a manufacturing…
Qualitative and Quantitative Host Cell Protein Analysis Using Mass Spectrometry
Host cell proteins (HCPs) originate from host organisms that are used to produce biopharmaceutical products. They are in-process contaminants that must be minimized during downstream process operations. According to regulatory agencies, the maximum permitted level of total HCP in a biopharmaceutical product is 100 ng per mg (100 ppm) (1). HCPs can decrease drug efficacy and pose a risk to patient safety because they can bring on undesirable immune responses. Thus, HCPs are a critical quality attribute that should be…
Multitiered Automation for Improved Efficiency of Bioprocess Analytics
The first biopharmaceutical, human insulin, was approved for use in 1982 (1). The biopharmaceutical market continues to exhibit healthy growth now, with the number of yearly patent applications increasing by 25% annually since 1995 (2). The total pharmaceutical R&D pipeline has more than doubled since the beginning of the century (Figure 1), much of that attributable to the biologics industry segment. As this industry has matured, new platform methods have emerged, and competition has increased. Consequently, the pressures of speed,…
Virus Segregation During Purification Processes: Calculation of Critical Potential Carryover of Viruses
Before a pharmaceutical product is introduced into humans, either in a clinical trial or as a marketed product, virus safety must be evaluated carefully. Virus safety normally is ensured using a three step complementary approach: selecting and testing cell lines and/or raw materials for the absence of viruses, testing the product at appropriate steps of production, and assessing the capacity of a production process to clear infectious viruses (1). The latter (also referred to as viral clearance) is the subject herein. Spiking studies are conducted to evaluate the capacity of a purification…
Setting Up a Rapid Mycoplasma Assay to Support Recombinant Protein Production
Octapharma AB (OAB) in Stockholm, Sweden, is the site for Nuwiq human recombinant factor VIII (FVIII), production. The drug is produced in a human cell line cultured in a perfusion bioreactor using a closed system (to minimize contamination) and proprietary serum-free medium without animal-derived components. In accordance with regulatory guidelines, cell banks and cell cultures used for production of biological products must be free of mycoplasma. Traditional mycoplasma testing is a growth-based method that represents a significant bottleneck in quality…
Methods on the Move: Addressing Method Transfer Challenges for the Biopharmaceutical Industry
Analytical method transfers are essential components of the current global biotechnology environment. Analytical method transfer can be defined as “a documented process that qualifies a laboratory (the receiving laboratory) to use a validated analytical test procedure that originated in another laboratory (sending laboratory), thus ensuring that the receiving laboratory has the procedural knowledge and ability to perform the transferred analytical procedure as intended” (1). The goal is to ensure that a method continues to perform in the validated state regardless…
Dual Sourcing of Protein A Resin to Mitigate Supply Chain Risk: A Comparative Study to Determine Equivalence
Protein A affinity chromatography is a well-established technology that is used extensively for large-scale purification of monoclonal antibodies (MAbs). With this mode of chromatography, very high product purity can be achieved in a single, relatively simple unit operation. A solution containing the target protein of interest is applied to a liquid-chromatography column at near-neutral pH, and one or more wash steps follow to lower product- and process-related impurities (1). Product is eluted through application of a low-pH buffer. Finally, the…