The therapeutic benefits of monoclonal antibodies (MAbs) have been demonstrated in recent decades with uncontestable success as treatments for human disease. Despite MAbs’ key features such as specificity, selectivity, and safety, the format has limitations (1, 2). Bispecific antibodies may overcome number of difficulties (3). Multiple formats of bispecific antibodies have been developed, although only the κλ-body is fully human and devoid of linkers or mutations. It requires no genetic modifications of heavy and light chains and results in bispecific antibodies…
Downstream Development
A UF–DF Screening System for Bioprocess Development: Efficient and Cost-Effective Process Fit and Scale-Up to Manufacturing
Ultrafiltration and diafiltration (UF–DF) of therapeutic proteins are performed in either tangential or crossflow mode using membrane filters. UF–DF plays a critical role in both downstream and upstream processes for the biopharmaceutical industry (1). In upstream production processes, classical tangential-flow filtration (TFF) or alternating tangential-flow (ATF) systems are used in high–cell-density perfusion for protein expression by cell culture (2). TFF is used in downstream processing for UF–DF and concentration of therapeutic proteins. TFF unit operations are common in protein purification…
eBook: Quality By Design for Monoclonal Antibodies — Establishing the Foundations for Process Development, Design Space, and Process Control Strategies
The quality by design (QbD) modernized approach to pharmaceutical development is intended to provide regulatory flexibility, increased development and manufacturing efficiency, and greater room to innovate as well as improve manufacturing processes within defined ranges without obtaining regulatory approval first. QbD is a systematic developmental approach that starts with a clear goal in mind and emphasizes understanding of how variability in both process and materials affects a final product (1). Historically, product quality has been assured either with end-product testing…
Host-Cell Protein Risk Management and Control During Bioprocess Development: A Consolidated Biotech Industry Review, Part 2
Even with increased understanding of host cell proteins (HCPs) and their potential risks, no practical approach has been made available for HCP risk management during bioprocess development. A BioPhorum Development Group (BPDG) team has identified common HCP-related risk factors and built a template for semiquantitative risk assessment during process development based on publicly available information. To this end, the BPDG HCP working team’s assay and knowledge-sharing experts have established a common HCP risk assessment tool and mitigation strategy to guide…
Host-Cell Protein Risk Management and Control During Bioprocess Development: A Consolidated Biotech Industry Review, Part 1
Host-cell proteins (HCPs) constitute a significant class of process-related impurities during biologics manufacturing. Due to their potential impact on product quality and efficacy as well as patient safety, the total amount of residual HCP in a biological drug substance generally is considered a critical quality attribute (CQA) that usually needs to be tested for during batch release (1, 2). It is both an “industrywide” common understanding and a regulatory requirement to remove HCPs from biologics to acceptably low levels that…
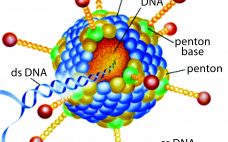
Development of a Freeze-Dried Ebola-Expressing Adenoviral Vector: Unexpected Findings and Problems Solved
In December 2013, a two-year-old child in Guinea became the first person to be killed by Ebola in the most recent outbreak. In March of the following year, that outbreak was declared in West Africa. By mid-2014, the World Health Organization (WHO) had declared it to be a public health emergency of international concern and urged pharmaceutical companies to accelerate their development of candidate vaccines. At the peak of the outbreak in 2014, more than 1,200 new cases of Ebola…
Rational Design of Liquid Protein Formulations: Application of Biophysical Stability Predictors and Descriptors to Reformulate Biotherapeutics
Successful development of liquid biopharmaceutical formulations requires careful assessment of the biophysical properties of the protein in solution, primarily focused on achieving optimal conformational and colloidal stability of the drug-substance molecule (1–11). It also involves extensive stability studies under stressed conditions. Using state-of-the-art biophysical tools for characterization of developed products, those studies are based on key biophysical descriptors and extended particulate characterization methods (subvisible particles in micro- and nano-size range) to deliver a stable product for market with a shelf…
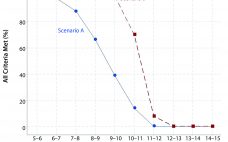
Statistical Assessments of Bioassay Validation Acceptance Criteria
Analytical linearity as well as assessments of precision and accuracy determine the range for a bioassay (1). USP <1033> recommends comparing confidence intervals (CIs) against target validation acceptance criteria in a bioassay validation exercise, but there are no clear guidelines for determining the criteria (2). Here I address several aspects of a bioassay validation, namely • Linearity (coefficient of determination (R2), slope, and intercept parameters) • Accuracy (%relative bias, %RB) • Precision (percent coefficient of variation, %CV) CIs for the…
eBook: Bioprocess and Analytical Laboratories — Proving the Power of Data in Drug Development
Analytics pervade the entire biopharmaceutical development process — from protein characterization through biomanufacturing process optimization to final-product formulation and clinical testing. Every technical article in BPI requires data to back up the statements made, whether the topic is upstream/production, downstream processing, product development, or otherwise focused. And never mind publishing: Even more detailed documentation is required for regulatory submissions. If a company can’t back up the choices made and results obtained in development, manufacturing, and testing of its biopharmaceutical product,…
The Multi-Mode Mimetic Ligand Library: A New Tool for Rapid Development of Downstream Processes
Recent developments in downstream processing of biomolecules — including continuous processing, bind–elute affinity capture, and flow-through polishing steps — have increased the need for greater selectivity from chromatography adsorbents. This has led to the introduction of a new generation of adsorbents: so-called “mixed-mode” or multimodal ligands. They provide greater selectivity and tolerance to process buffer composition than either ionexchange chromatography (IEC) or hydrophobic-interaction chromatography (HIC) alone can provide. Learn more in this Special Report from Steve Burton, Chief Executive Officer…