Geert Van Raemdonck (global field support expert at PharmaFluidics) and Koen Sandra (scientific director of the Research Institution for Chromatography, RIC) teamed up for a 10 October 2019 “Ask the Expert” webinar to introduce micro Pillar Array Column (μPAC™) technology for liquid chromatography–mass spectrometry (LC–MS) for host-cell protein (HCP) detection. Van Raemdonck explained that μPAC technology approaches chromatography differently than does packed-bed technology. Microfluidic channels with arrays of free-standing pillars are etched lithographically into a silicon wafer. The resulting permeability…
Downstream Development
Host-Cell Protein Analysis to Support Downstream Process Development: A High-Throughput Platform with Automated Sample Preparation
In the past few years, increasing numbers of biotherapeutics have been approved for market (1). Among all the regulatory concerns for commercial biotherapeutics, host-cell proteins (HCPs) are a major class of process-related impurities that remains a critical quality attribute (CQA) for bioprocess development because of associated risks to product quality, safety, and efficacy. HCP identification, clearance, assay setup, and process control are critical points for health authorities, and many guidelines aim for better control of HCP content in final biologic…
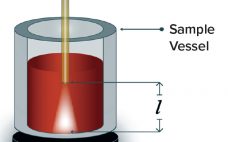
Viral Nanofilter Integrity: Using Variable-Pathlength UV-Vis Spectroscopy for the Gold Nanoparticle Test
Viral filtration (VF) using nanofilters removes endogenous and/or adventitious viruses from biologic drug-substance manufacturing processes (1). The gold particle test (GPT) is performed as part of postuse integrity testing — to complement postuse leakage testing — for cellulose filters such as Planova 20N filters from Asahi Kasei Corporation. First, a proprietary gold-colloid solution matched to the filter type (e.g., 20N) is filtered through the test article. That filter’s pore-size distribution can be assessed using spectrophotometric absorbance readings of the integrity-test…
Adenovirus Downstream Process Intensification: Implementation of a Membrane Adsorber
Historically, companies developing vaccines have used attenuated pathogens, inactivated infectious agents, or antigenic constituents purified from pathogenic sources. In the past 20 years, technological advances such as recombination and viral vectors, have enabled development of vaccines against diseases with previously no available treatments (1). Viral vectors have become one of the most rapidly evolving and promising fields in vaccinology and regenerative medicine. In addition to preventing infectious disease, they have a broad range of potential applications, including treatment of hereditary…
Rapid Implementation of Novel Affinity Purification: Manufacture of Commercial-Scale Next-Generation Antibody Therapies
The rapid and cost-effective production of conventional monoclonal antibodies (MAbs) for clinical trials and commercial supply has contributed toward their wide adoption. Production processes have become more efficient because common purification processes are being used across structurally similar MAbs during key steps of process development and manufacturing. Such successful platform approaches can remove unwanted impurities and are stable across processing conditions, irrespective of the MAb being purified. In addition, they are readily available at the required volume to support large-scale…
CMC Development Platforms and Outsourcing to Reduce Timelines
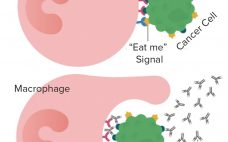
Forty Seven is a company developing novel therapies based on anti-CD47 and other immuno-oncologies. CD47 is called the “don’t eat me” signal that cancer cells give out to escape elimination by the body’s immune system. Qinghai Zhao, vice president of technical development and manufacturing, is one of the scientists working on the company’s magrolimab (5F9) monoclonal antibody that is designed to block the binding of the CD47 signal to the cell receptor SIRP-α while boosting the “eat me” signal that…
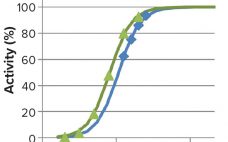
Apparent Matrix Effects in an Iduronate 2-Sulfatase Specific Activity Assay
The recombinant fusion protein SHP631 consists of a chimeric monoclonal antibody binding to human insulin receptor and iduronate-2-sulfatase (I2S). This product is being developed as an enzyme replacement therapy to treat cognitive symptoms of Hunter’s syndrome. Because the current therapy (idursulfase, brand name Elaprase from Shire) cannot cross the blood–brain barrier (BBB), SHP631 is being developed to do so, enabling the presence of I2S in the brain. The enzymatic activity of this molecule is measured using the substrate 4-methyl umbelliferyl-α-L-idopyranosiduronic…
Developing an End-to-End Scale-Down Model for a Commercial-Scale Downstream Process: Enhancing Technology Transfer Efficiency
Large and complex protein molecules used as therapeutic agents are manufactured in a series of process steps that start with thawing of cell-bank vials and finish with filling and packaging (Figure 1). The cost and complexity of commercial-scale biomanufacturing processes make them prohibitive to troubleshoot or experiment at full commercial scale. Biopharmaceutical companies routinely use scale-down models (SDMs) of licensed commercial-scale processes to evaluate raw material changes, process improvements, and deviations (1) (Figure 2). Here, we outline some considerations in…
Continuous Chromatography: Experts Weigh in on the Possibilities and the Reality
Discussions of continuous processing in the biopharmaceutical industry are an important part of current efforts toward intensifying bioproduction and bioprocessing. Biomanufacturers are looking at all components of their development and manufacturing processes for ways to reduce the size of their facilities, lower costs, and increase speed and flexibility of operations. Increasing options for and availability of single-use technologies have been major enablers of myriad attempts to improve efficiencies. Although the general consensus may still be that single-use components are more…
Aspects of Acceleration: Biomanufacturers Need Smart Strategies to Speed Products to Market
No matter what the industry, it’s widely accepted that slow-moving companies give their nimbler competitors an advantage, allowing them room to dominate the market even if their products are not superior. “Me-too” products and their sponsors often are seen as followers rather than leaders — even if they offer improvements over what is already available. Fast movers are flexible and adaptive to a dynamic business environment. They capitalize on opportunities and navigate risks and challenges by responding quickly to changes…