The successful commercialization of a biopharmaceutical product begins with a robust and productive cell line. Inefficient cell-line development (CLD) can lead to costly delays and roadblocks. For that reason, small, new, and virtual companies — and even established and mid-size companies — often seek the support of outsourcing partners to develop their cell lines. Outsourcing CLD activities can ease many pressures associated with manufacturing new biotherapeutics. The benefits of outsourcing CLD and associated processes include access to specialized expertise and…
Cell Line Development
Contractor Perspectives: Best Practices for Transfer, Handling, Testing, and Storage of Cell Banks
For comments about how contract development and manufacturing organizations (CDMOs) manage their cell-banking quality assurance (QA) practices. I contacted long-time member of BPI’s Editorial Advisory Board Scott M. Wheelwright, PhD, for his perspectives. Wheelwright brings many years of experience to this discussion, with insights into the evolution of technologies and practices extending back to the early launch of the biopharmaceutical industry. Currently, he provides consulting support for companies with manufacturing and sourcing in China and other Asian countries. He also…
Cell-Line Development: Accelerating Antibody Discovery By Monitoring Titer and Glycosylation with the Octet Platform
Cell line development involves the screening of thousands of clones in an effort to find the few optimal clones that are stable, grow as expected, and produce high yields of the bioproduct. The time it takes from engineering an optimal cell line to the production of the target biologic can be prohibitive and may differ from molecule to molecule. While expression-level analysis like titer screening is carried out early, other critical quality attributes (CQAs) such as glycan characterization are often…
Cell Banking in the Spotlight: Advising Biologics Developers About Cell Bank Preparation and Characterization
Living cells are at the heart of biotechnology, and cell lines for production and testing of biopharmaceuticals are highly valuable assets. The process of banking cells generally moves from development of a research cell bank (RCB) based on a clone of interest to establishment of a master cell bank (MCB), from which working cell banks (WCBs) can be produced. Especially for biotechnology startups, preparation of an MCB can involve a significant jump from work performed in standard laboratory conditions to…
Optimizing Cell Line Development for High-Quality Biologics
For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess…
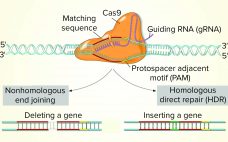
Use of CRISPR and Other Gene-Editing Tools in Cell Line Development and Engineering
While the role of biologics in treating human diseases has evolved dramatically over the past decade, so has genetic engineering. Rational genetic engineering to enhance biotherapeutic proteins has become a reality catalyzed by publication of the genome sequences of multiple Chinese hamster ovary (CHO) cell lines. Novel “designer” CHO cells modulate posttranslational modifications (PTMs) of recombinant proteins by genome editing, and it is now possible to knock-in or knock-out genes of yeast and mammalian cells precisely (within one DNA base…
Plant-Cell Cultures and Cell Lines for Recombinant Protein Expression
Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (1, 2). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity…
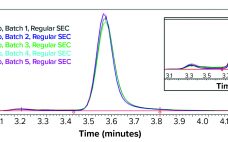
Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization
Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin G (IgG) product integrity in real time during a bioreactor run, especially…
The Upstream Perspective: Taking Efficiency Beyond Cell-Line Development
With 20 years of experience in the biopharmaceutical industry — at Genentech, Applied Biosystems, Cell Genesys, Cellerant Therapeutics, and Bayer — Yuval Shimoni has written frequently for BioProcess International on a number of production topics. Those have ranged from process improvements and bioreactor scale-down validation, to raw materials management, to addressing variability and virus contamination events. For this featured report, we discussed hardware and instrumentation, quality by design (QbD) and related approaches, and other strategies that can take expediting upstream…
Anticipating Cell-Line Challenges to Drive CMC Readiness
Development of a safe and high-quality Chinese hamster ovary (CHO) cell line is of paramount importance for the chemistry, manufacturing, and controls (CMC) portion of studies that support investigational new drug (IND) applications (1, 2). Desirable attributes of a CHO cell line include its ability to produce high titers of biotherapeutic proteins facilitate quick recoveries and selection processes maintain phenotypic and genetic stability throughout in-vitro aging of a culture. A CHO cell line also should be scalable to high-capacity culture…