Astrea Bioseparations has a well-established modular program to support customer projects from small to large scales with ligands, adsorbents, and chromatography columns that design purity into each process. Demand for increased productivity in biopharmaceutical manufacturing has placed new pressure on downstream purification operations. For recombinant proteins and monoclonal antibodies (MAbs), such pressure stems from significant gains in upstream productivity, particularly from high titers produced using increasingly efficient cell-culture systems. For viral vectors used in gene and gene-modified cell therapies and…
2022
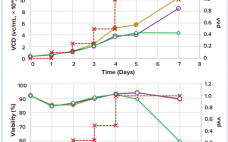
High-Yield Production of rAd26-S for Sputnik V Vaccine Component I: An Optimized Process in a Scalable Shaken Bioreactor
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other…
Gain Control of Culture Conditions: Technology for Sustained Delivery of Recombinant Proteins
Growth factors, added at the precise time and concentration to in vitro cultures are essential to control cell proliferation and differentiation. When added to a culture vessel, however, the concentrations of these signaling proteins rapidly decline, altering both levels of individual growth factors and the ratio of factors for cell signaling. When growth factors are replenished by exchanging the medium, concentrations peak, resulting in an ebb and flow of growth factor levels resulting in mixed signaling that can lead the…