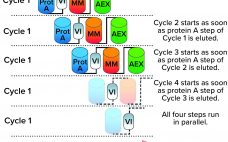
The cell and gene therapy (CGT) industry has grown out of disparate groups of developers, suppliers, and service providers. Players in the sector must collaborate if we expect to commercialize new therapies at scale. Despite concerted efforts in the field, however, the critical question of how to automate CGT production has yet to be answered. Without automated processes in place, the sector risks spiraling costs and operational inefficiencies that could inhibit patient access to much-needed therapies. Understanding such concerns, several…
2022
Ask the Expert: In-Line Concentration Measurement for Adaptive Control of Downstream Processes
In-line process analytical technologies (PATs) hold much promise for enhancing control of biopharmaceutical manufacturing processes and, in turn, increasing process quality and reproducibility. During a January 2022 presentation, Ramsey Shanbaky (associate director of bioprocess applications at Repligen) described how his company’s CTech FlowVPX system for in-line protein concentration measurement enhances monitoring of downstream processes. Shanbaky’s Presentation Scientists often use ultraviolet–visible spectroscopy (UV-vis) to measure protein concentration in a drug substance (DS) or product (DP). Most UV-vis instruments determine that by…
eBook: Potency Bioassays — Development, Trending,
Transfer, and Automation
Bioassay development is a complex process that must be undertaken with great rigor and attention to detail. Potency testing experts use a range of methods including cell-based and binding assays. Consistency and reliability of results over time are paramount. Well-developed and -characterized methods are the end result of much phase-appropriate development work that goes on in parallel with bioprocess and biotherapeutic product development. This eBook begins with BPI senior technical editor Cheryl Scott’s report from the Biopharmaceutical Emerging Best Practices…
Navigating New Options for Commercial-Scale Biopharmaceutical Production
Scalability remains a critically important topic for biopharmaceutical companies. For conventional protein products, the strategy once was straightforward: Drug makers would scale up, beginning with cultures in flasks and roller bottles to grow enough cells to inoculate laboratory-scale (often glass) bioreactors, then again to pilot- and commercial-scale, stainless-steel, stirred-tank bioreactors. At their highest volumes, such reactors can handle tens of thousands of liters of cells and growth media. If a drug developer did not have the requisite equipment to scale…
Boosting Yield and Preserving Quality: Increasing Production of Induced Pluripotent Stem Cells for Cell Therapy and Regenerative Medicine Applications
Induced pluripotent stem cells (iPSCs) are promising starting materials for several clinical and industrial applications in cell therapy production, drug discovery, and in vitro screening. But as I learned from Maxime Feyeux (cofounder and chief science officer of TreeFrog Therapeutics in Bordeaux, France), such cells are a relatively new option in the toolbox of cell therapy developers. Advanced-therapy products can require controlled proliferation and differentiation of millions or even billions of iPSCs depending on the clinical indication, he pointed out,…
Reducing Downstream Scale-Up Needs: Advances Toward Continuous Downstream Processing
The biopharmaceutical industry generally acknowledges that upstream and downstream aspects of drug-substance manufacturing are experiencing a capacity mismatch. Today, many recombinant proteins can be produced at expression titers of 3 g/L, with some yields exceeding 10 g/L. Such titers represent 100-fold increases in production capability compared with values from twenty years ago (1, 2). Increases in cell-culture density and improvements to perfusion-mode bioreactor systems hold promise for increasing yields further still. Such developments, combined with the broad availability of concentrated…
Scaling AAV Production: Easing the Transition from Laboratory Scales to Commercial Manufacturing
Adenoassociated virus (AAV) has emerged as the leading vector for gene therapy delivery. Compared with options such as lentivirus and adenovirus, AAV exhibits a strong safety profile because it has low pathogenicity and requires a helper virus to replicate. AAV is also capable of long-term gene expression, and it can infect both dividing and nondividing cells (1–5). Developers of advanced therapies have found such advantages to be quite attractive. As of January 2021, two gene therapy products have gained US…
March 2022: From the Editor
As you receive this issue, Informa’s BPI West conference is taking place at the San Diego Convention Center on 14–17 March 2022, with live digital panel discussions the following week. People appear to be cautious but eager to get back to live-conference attendance. BPI’s senior technical editor, Cheryl Scott, will make the journey down there to represent us this year, so if you plan to be there, please take a moment to visit with her. BPI West was the first…
Orphan Drug Designation: Securing the Significant Benefits
When preparing an application for orphan drug designation (ODD) in the European Union (EU), you need to consider key eligibility criteria that are different from those required by the US Food and Drug Administration (FDA). One factor is justifying the “significant benefit” (defined below) of your drug product over existing therapies for an orphan condition. Significant benefit contributes considerably in securing a successful ODD application if the correct approach is taken — as described in two European Medicines Agency (EMA)…
Maximizing European Market Access: Guidance for Young Biopharmaceutical Companies
Achieving efficient and profitable market access for next-generation pharmaceutical products is extremely challenging. The number of drug launches is rising every year, taking competition levels higher with them. And because these novel products tend to be more tightly targeted to smaller patient populations than the “blockbuster” drugs of old, their pricing/reimbursement terms need to be tailored to match. This is especially the case with highly complex biologic drugs, which typically are expensive to research and develop. Below I offer a…