Despite safety-related setbacks in the early 2000s, adenovirus (Ad) again is gaining traction as a vector for advanced therapies and vaccines. Thus, Ad production is accelerating. In November 2021, Hana Jug (project manager in process development for viral vectors and vaccines at BIA Separations, a Sartorius company) described how her company’s updated platform process for Ad vectors could enhance their purification, maximizing recovery of critical vaccine components. Jug also highlighted BIA’s abilities to supply chromatographic columns consistently and to support…
January-February 2022
Ask the Expert: Rapid Determination of AAV Capsid Titers at Early Manufacturing Stages
During a December 2021 presentation, Matthew Lotti (senior research associate in analytical development at Ultragenyx) highlighted difficulties with determining adenoassociated virus (AAV) capsid titers during gene therapy production. With support from Gyros Protein Technologies, Lotti spoke about his team’s development of a method for measuring AAV titers using a Gyrolab xPand immunoassay system. Lotti’s Presentation Simple analytical methods such as UV spectrophotometry measure capsid titers most effectively after virions have undergone purification. To test samples from earlier process stages, Ultragenyx…
An Ethical Option for Shelved Drugs
A key priority in today’s investment world is corporate adherence to environmental, social, and governance (ESG) requirements. A company’s ESG score serves as a marker of the organization’s values and as a disclosure mechanism for investors to consider. Many companies now consider ESG scores when making strategic choices, and my group has identified a tangible option. In 2019, the Children’s Tumor Foundation and CureSearch for Children’s Cancer, launched the Bridge initiative (1) in partnership with FasterCures, a division of the…
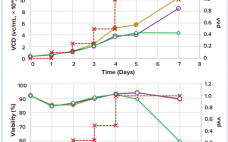
High-Yield Production of rAd26-S for Sputnik V Vaccine Component I: An Optimized Process in a Scalable Shaken Bioreactor
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other…
Gain Control of Culture Conditions: Technology for Sustained Delivery of Recombinant Proteins
Growth factors, added at the precise time and concentration to in vitro cultures are essential to control cell proliferation and differentiation. When added to a culture vessel, however, the concentrations of these signaling proteins rapidly decline, altering both levels of individual growth factors and the ratio of factors for cell signaling. When growth factors are replenished by exchanging the medium, concentrations peak, resulting in an ebb and flow of growth factor levels resulting in mixed signaling that can lead the…