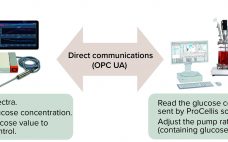
The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be “tested into” a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development. Monitoring of product quality attributes (PQAs)…
2021
Considerations for Developing Inhaled and Nasally Delivered Biologics
Inhalable drug formulations show much promise for improving biopharmaceutical delivery. But as Ashleigh Wake (director of business development at Intertek) explained to Ask the Expert attendees in September 2021, such formulations necessitate development of complex quality control (QC) analytical methods. Wake highlighted special considerations for characterizing and controlling the stability and potency of inhaled and nasally delivered biologics. Wake’s Presentation Wake emphasized that nasal and inhaled biological products necessitate QC strategies to control both a protein product and its delivery…
eBook: Creating CMC Strategies for Pandemics and Beyond — Perspectives on the Impact of the COVID-19 Pandemic
The COVID-19 novel coronavirus pandemic has highlighted the biopharmaceutical industry’s need to create and implement chemistry, manufacturing, and controls (CMC) strategies that can expedite drug development during times of crisis. This bold topic was the premise of the 2021 CASSS Well-Characterized Biotechnology Products (WCBP) forum titled “Special Edition: Creating CMC Strategies for Pandemics and Beyond.” Held virtually on 25–28 January and 1–4 February 2021, the eight-day event addressed manifold considerations for SARS-CoV-2 virus prevention, diagnosis, and treatment. Five hundred attendees…
eBook: Cell and Gene Therapies — Optimization Strategies for Processing and Potency
Although relatively new to the biopharmaceutical industry, cell and gene therapy development and manufacturing are advancing rapidly. At Informa Connect’s September 2021 Cell & Gene Manufacturing & Commercialization Conference and Exhibition, held in Boston and online, presentations reviewed concerns that arise when processing complex therapies and highlighted some innovative strategies for surmounting those obstacles. Most of those approaches described during the event used data-driven solutions, with each step building on the information gained from the previous one. High-throughput technology platforms…
Advancing Sterile-Connection Technology for Low-Volume Fluid Transfer
Biomanufacturers traditionally have relied on laminar-flow hoods or thermal tube welding to establish connections for small-format bioprocesses, including those for cell and gene therapy (CGT) production. But such strategies can introduce processing risks and can pose operational challenges. In October 2021, Troy Ostreng (senior product manager at CPC) described how his company’s MicroCNX sterile connectors could meet biomanufacturers’ needs for low-volume applications. Ostreng’s Presentation Welding tubes and forging open connections under laminar hoods raise several disadvantages for small-format bioprocesses. Processing…
eBook: Vaccines Revisited — The Past, Present, and Future of Nucleic-Acid Vaccine Production
The quarterly BioProcess Insider eBook series launched in 2021 to investigate specific modalities and business strategies driving the biopharmaceutical sector based upon trends and discussion points presented in the Insider’s pages. Two of the four quarterly publications — this one included — have focused on the vaccine industry. If the series had launched in 2019, cell and gene therapies might have warranted a double edition, or perhaps investments in antibodies and antibody fragments would have driven a bispecific focus. Five…
A Practical Approach to Clinical Trial Diversity
A 2021 study in the Journal of the American Medical Association found that minority groups were underrepresented in US-based vaccine clinical trials, with white people accounting for ~80% of participants (1). Although researchers broadly acknowledge that trial representation should mirror the many different populations who could benefit from a drug, lack of diversity continues to hamper clinical-trial effectiveness. To address this problem, the pharmaceutical industry must increase awareness about clinical trials and reduce barriers to participation in them. Benefits of…
Optimizing the AAV Transfection Process in Suspension Cells
Given the potentially curative nature of gene and gene-modified cell therapies and the successful launches of several such products over the past five years, markets and investments are growing significantly in the sector. In the United States alone, the US Food and Drug Administration has granted approval for several genetic therapies, including Strimvelis (autologous CD34+, developed by GlaxoSmithKline and later sold to Orchard Therapeutics) in 2016 Luxturna (voretigene neparvovec, Spark Therapeutics), Yescarta (axicabtagene ciloleucel, Kite Pharma/Gilead), and Kymriah (tisagenlecleucel, Novartis)…
Viral Safety of Viral Vectors:
Special Concerns Arise When the Virus Is the Product
As anyone who has focused on host-cell proteins as process contaminants can tell you, trying to purify a specific type of molecule from a large mixture of many similar molecules is like trying to find a few particular needles in a huge pile of varied needles. The same could be said for purifying viral vectors from cell culture fluids. When viruses are the products, unwanted viruses are contaminants that must be separated away — or better yet, prevented from being…
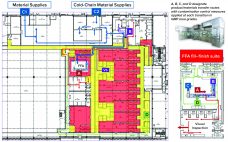
Formulation, Fill and Finish of Lentiviral Vectors: Part 1 — Case Study in Facility and Process Design
Over the past few years, Oxford Biomedica Ltd. (OXB) has developed and implemented a fill–finish platform (“Oxbox,” Figure 1) at its viral vector processing facility in the United Kingdom. The facility includes four segregated bulk viral-vector drug substance (VS) suites, where closed systems and bioburden control processes apply, and two viral-vector drug product (VP) fill–finish suites that apply aseptic processing, with space for expansion by scale-out as product output demand increases. Segregated suites enable the facility to process different viral…