A reasonable estimate of long-term variation for a biopharmaceutical product critical quality attribute (CQA) can be challenging to justify, especially in the early stages of a product’s lifecycle when only limited data are available. However, if the combination of product and analytical method reasonably can be matched with historical data, prior knowledge can provide an estimate of a value. This variation estimate could be used to assist in risk assessments related to continued process verification (CPV) activities, including control charting…
2021
Ask the Expert: FPLC Column Selection Considerations
On 10 November 2020, BPI presented an “Ask the Expert” webinar with Dan Yukon (head of North American and global SNAP product sales at Astrea Bioseparations) on considerations for selecting analytical fast-protein liquid chromatography (FPLC) columns. With many options on the market, deciding which type and brand to use can be difficult. To help take out the guesswork, Yukon addressed a number of topics, including pressure and volume considerations; column configuration; materials of construction; frit type, design porosity, and mounting;…
Ask the Expert: Centrifugation Guided By Optical Sensors Enables Efficient, Reagent-Free Cell Separation
Ben Josey, PhD (field application scientist at Corning Life Sciences), joined BPI on 3 December 2020 to deliver an “Ask the Expert” presentation about using optical-sensor–guided centrifugation for cell therapy development. Cell-separation techniques fall into four basic categories: filtration, centrifugation, affinity purification, and emerging methods such as microfluidics and acoustofluidics. Selecting the technology best suited to an application requires careful balancing of method precision and process efficiency, the latter of which includes factors such as batch size, time, labor, and…
Ask the Expert: New and Improved Analytical Methods for Traditional and Unique Modalities
On 10 December 2020, BPI presented an “Ask the Expert” webinar with Jason Sterling, PhD (principal scientist and project director in analytical and formulation resources), and John Rockwell (group leader) of Catalent Pharma Solutions. Biophysical characterization is critical to understanding the makeup and behaviors of biologic therapies and vaccines both early in development and throughout scale-up for manufacturing. As biologics become more complex in structure and as scientists improve their understanding of the effects of structure on stability, efficacy, and…
Ask the Expert: Developing Strategic Analytical Programs for Therapeutic Peptides
Ashleigh Wake began her 15 October 2020 “Ask the Expert” presentation by pointing out that peptide products are manufactured in a “regulatory vacuum.” Peptide-product developers must be strategic in designing characterization and quality control (QC) programs. Wake reviewed available methods and explored key considerations for developing phase-appropriate analytical controls. Wake’s Presentation Because peptides overlap small- and large-molecule drugs in size, regulatory expectations differ by product size and clinical indication. Thus, analytical programs should be designed around critical quality attributes (CQAs)…
COVID-19 As a Catalyst for Changing Orphan-Drug Regulations
A long-awaited (and for months withheld) evaluation of the European Union’s Orphan Drug Regulation (ODR) finally reached the interested public in August 2020 (1). Its publication during the public consultation of the European Commission’s “Pharmaceutical Strategy” was well planned because the latter discusses policies on access, availability, and affordability of new medicines. However, the ODR evaluation shows not only that the intentions behind the legislation have not been fulfilled, but also that its generous incentives (extension of market exclusivity and…
eBook: Vaccines — COVID-19 Invigorates a Stagnant Industry
The SARS-CoV-2 novel coronavirus has galvanized what was a stagnant and oligopoly-run vaccine industry. In this inaugural BioProcess Insider eBook, the first of four to be published in 2021, founding editor Dan Stanton explores economic and technical conditions that until now have hampered innovation in vaccine development and discouraged market entry for emerging biotechnology companies. Leveraging commentary from vaccine industry experts and analyzing the range of emerging vaccine modalities, Stanton surveys how industry responses to the COVID-19 pandemic are fostering…
Exciting Starts for New Players and Platforms: Nucleic-Acid Vaccines Prepare for Their Commercial Debut
Although vaccine platforms based on messenger RNA (mRNA) are enjoying the limelight in the wake of emergency authorizations of products from Pfizer–BioNTech and Moderna, DNA vaccines are poised to make their own commercial debuts soon. The World Health Organization (WHO) reports that six of the 48 candidate vaccines against SARS-CoV-2 that remain in clinical trials are DNA-based products, as are 14 others in preclinical study (1). I spoke with Hong Jiang (cofounder and chief operating officer of Aegis Life, Inc.)…
Cold-Chain Validation: Emerging Vaccines for COVID-19 and Beyond Require More Extensive Evaluation
In the wake of fast-track approvals for Pfizer’s and Moderna’s respective SARS-CoV-2 vaccines, now begins the largest immunization campaign in world history. Its success will depend not only on the products’ safety and efficacy, but also on several mass-distribution programs requiring significant cold-chain infrastructure. The public has become acutely aware of the Pfizer vaccine’s demanding cryostorage specifications, generating considerable anxiety about how mass distribution will happen. Behind the scenes, however, cold-chain engineering companies such as Modality Solutions have worked alongside…
Enhancing Vaccine Platforms: Computational Models Accelerate Development, Manufacturing, and Distribution
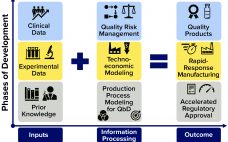
Pandemics such as the current COVID-19 outbreak pose tremendous healthcare and economic challenges. Vaccines hold promise for controlling pandemics; however, substantial challenges come with pandemic-response vaccine development, manufacturing, distribution, and administration. To address those now, many companies are using rapid-response vaccine-production platform technologies. Computational modeling tools could help further accelerate development of those technologies, increase production and distribution efficiencies, and reduce costs and risks once vaccine platforms are fully developed and validated. To those ends, a set of modeling methodologies…