Cell-based potency testing provides quantitative data concerning a drug’s biological activity. Thus, it plays an essential role in biopharmaceutical quality control (QC), good manufacturing practice (GMP) product release, comparability determination, and stability testing for both drug products and drug substances. Potency is a critical quality attribute (CQA) often scrutinized by regulators and reviewers. Test methods are specific to a drug’s mechanism of action (MoA) and should be validated to internationally harmonized regulatory standards (1). The options preclude applying a simple…
2021
HCP Assay Development: Managing Risks with Evolving Technologies
Host-cell proteins (HCPs) are major impurities of concern in biomanufacturing. When present in drug formulations, they can reduce efficacy (by compromising product stability), introduce toxicity, and increase a recipient’s risk for long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are important parts of developing new biological drugs — to fulfill regulatory guidelines and to ensure patient safety through product quality. HCP populations can be both complex and structurally diverse, and some changes in upstream culture conditions can affect…
Stability Testing: Monitoring Biological Product Quality Over Time
Many physical and chemical factors can affect the quality, safety, and efficacy of biopharmaceutical products, particularly after long-term storage in a container–closure system that can be subject to variations in temperature and light, as well as agitation with shipping and handling. Proteins are inherently complex physiochemically, from their primary amino acid sequences to their higher-order structures, and they require specific conditions to maintain their integrity and functionality. Advanced biological therapies can be even more complicated and particular about their environments.…
March 2021: From the Editor
In my short time as BPI’s resident novice, I have been impressed with this industry’s interdisciplinarity and collaborative spirit. Executives tell me that biopharmaceutical companies have not always been so multifaceted in their expertise or so willing to forge partnerships across organizations and over the industry–academia divide. I am lucky to have started working with you when I did late in 2019. That is not to regard the biopharmaceutical industry with rose-tinted glasses. Many elements of biomanufacturing remain closely guarded…
Embracing Innovation in Biomanufacturing
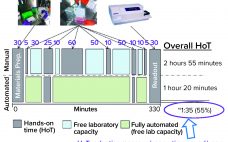
Innovations in bioproduction of therapeutics over the past 20 years have led to impressive improvements in product yield, process controls, and manufacturing safety. Industry 4.0 concepts have been embraced across the bioprocess industry and are leading to better bioprocess control through process automation, “big data” and data analysis, process simulations, the industrial internet of things (IIoT), cybersecurity, the cloud, blockchain/serialization, and additive manufacturing. Such advances help to ensure that a process results in the same outcome every time. As Sean…
The EU MDR Deadline Delay: What Does It Entail for Pharmaceutical Companies?
The life-sciences industry has been working hard to meet the deadline for compliance with the European Union’s Medical Device Regulation (EU MDR, 2017/745) (1). Doing so has been a challenging journey for many companies. Therefore, the full-year postponement of the final application date has been a welcome development, particularly in view of the new and extraordinary challenges stemming from the COVID-19 global health crisis. The extension has instigated other important changes, so it is critical that life-science businesses familiarize themselves…
Emerging Strategies for Drug Product Comparability and Process Validation: Part 1 — Analytical Tools and Drug Product Comparability
Process validation is a key part of the development and manufacture of all approved drug products, but its completion can be a daunting task. At a two-day CASSS CMC Strategy Forum held in July 2016 in Gaithersburg, MD, speakers and attendees addressed the many technical, practical, and regulatory facets of drug product process validation and comparability. In part 1 of this report, we summarize the key discussion points of the first day, which focused on analytics and comparability. Session One:…
Customized Yeast HCP Quantification with Biolayer Interferometry Using a Horseradish Peroxidase Substrate
Biopharmaceuticals are the largest group of drugs under development (1), and the demand for new and safe drug products is high. The most common bacterial and mammalian cell lines for production are Escherichia coli, Chinese hamster ovary (CHO) cells, and yeast. During a production bioprocess, a cell line expresses not only the molecule of interest, but also host-cell proteins (HCPs). They are considered to be impurities in a final drug product because they can affect the efficacy and safety of…
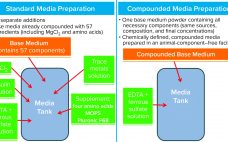
Compounded Media Powder Streamlines Cell Culture Media Preparation Operations
Cell culture medium is critical to cell growth, metabolism, and protein expression. It provides for optimum pH, osmolality, and nutrients in an environment that is essential for cell survival, growth, and expression of proteins and/or metabolites and drug-substance modalities of interest (1). A complete medium typically contains basic nutrients such as carbohydrates, amino acids, lipids, salts, vitamins, trace metals, growth factors/hormones (e.g., insulin), antishear factors, and other chemicals that facilitate cell growth and protein expression and may stabilize recombinant protein…
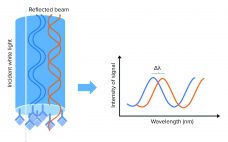
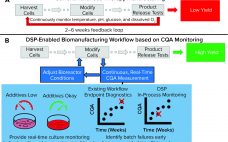
Improving Cell Manufacturing Outcomes Using In-Line Biomarker Monitoring
Cell-based advanced therapies are changing modern medicine dramatically. Immunotherapies such as chimeric antigen receptor (CAR) T-cell therapies are treating different forms of cancer. Gene therapies are reversing the course of inherited diseases, and tissue-engineered medical products are restoring, maintaining, and replacing damaged organs (1–4). The development of new advanced therapies is booming. As of January 2020, the US Food and Drug Administration (FDA) has reported more than 900 investigational new drug (IND) applications for cell and gene therapy products. However,…