Adoption of single-use systems (SUS) has increased steadily over the past few years driven by an increased focus on manufacturing biologics that require smaller scales than blockbuster drugs. Although SUS provide many benefits, some factors need to be considered when implementing them in biopharmaceutical production. With the regulatory landscape intensifying worldwide, drug manufacturers are expecting more data from suppliers to support the purity claims of supplied products, especially related to the purity of their product-contact surfaces. In this article, we…
January-February 2021
A Universal Assay Determination Method for Antisense Oligonucleotides: A New Slope Spectroscopy Method
Antisense oligonucleotides (ASOs) are short, synthetic, single-stranded oligodeoxynucleotides that can alter RNA and reduce, restore, or modify protein expression through several distinct mechanisms. ASO technology has become an important drug discovery platform for most major pharmaceutical companies. To date, six antisense drugs have been approved by regulatory agencies to treat diseases spanning viral infections, hyperlipidemias, and neurological diseases. More than 50 additional ASO drugs are in clinical trials. For an ASO drug product, an assay of its active pharmaceutical ingredient…
Ask the Expert: Enabling Digital Chromatogram Review to Streamline Column Evaluations
Chromatogram review enables evaluation of packed-bed chromatography processes. Typically, that method entails paper-based, qualitative comparison of a profile obtained during a run with another from a reference batch. Such a protocol can be time consuming, resource intensive, and susceptible to operator error. Martin D. Jensen (senior engineer at Fujifilm Diosynth Biotechnologies, FDB) delivered an “Ask the Expert” presentation on 20 October 2020 to describe advantages that his company gained by transitioning from paper-based to digital chromatogram review. Jensen’s Presentation Chromatogram…
Ask the Expert: Revealing Cell Secrets Using Optical Sensors in Upstream Processing
Upstream operations can benefit significantly from real-time, in-line testing of cell-culture conditions. Yet as Jake Boy (senior application scientist at Scientific Bioprocessing Inc., SBI) observed in his 5 November 2020 “Ask the Expert” webcast, traditional electrochemical probe sensors do not perform well in T-flasks, shake flasks, Petri dishes, and microfluidics devices. Boy described how fluorescence-based optical sensors can glean valuable data from small culture devices, assisting with research into cellular growth. SBI’s Presentation Boy began by highlighting successful applications of…
Ask the Expert: Derisking INDs with Unrivaled Monoclonality Assurance in Cell-Line Development
On 1 December 2020, BPI presented an “Ask the Expert” webinar with Tanner Nevill (vice president of program management at Berkeley Lights). Monoclonality assurance is a central regulatory requirement for all cell lines producing biologic therapies. Well-plate imaging is the “gold standard” for confirming that a cell line originates from a single cell, but it is labor intensive and prone to errors in the form of “ghost cells” and debris that look like cells. Using the Berkeley Lights Beacon system, cells…
Therapeutics Innovation in a Pandemic Era
Biopharmaceutical companies have demonstrated a rapid and powerful response to the COVID-19 pandemic, with innovators leading the way. A rush of investor money has been pouring into companies with the best odds of making it to the vaccine finish line. But such heavy investments in one sector lead some industry experts to wonder about the financial support of research into cure pathways for other serious diseases such as cancer, multiple sclerosis, and Alzheimer’s disease. When the pandemic abates with vaccines…
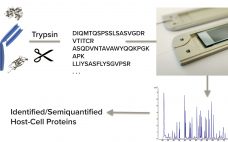
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…