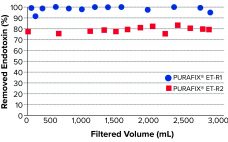
PURAFIX ET-R depth-filter sheets are used for efficient and economical reduction of endotoxins from pharmaceutical liquids. Removal of endotoxins is one of the most important and challenging steps in biopharmaceutical processes. Endotoxins — more precisely, lipopolysaccharides (LPS) — are extremely heat and pH stable and therefore withstand sterilization. Purification usually is performed by applying chromatography as a polishing step, which is costly and time consuming. By reducing endotoxin load at an early stage of a clarification process, subsequent purification steps…
2020
Enabling Digital Chromatogram Review for a Faster and More Reliable Operation
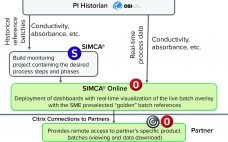
Chromatogram review is a monitoring method used to verify process performance in packed-bed chromatography processes. By observing key process parameters such as chromatography column outlet conductivity or UV absorbance, it is possible to identify the signs of a poorly packed column, resin degradation, or equipment malfunction. Therefore, chromatogram review is implemented as an in-process control (IPC) to decrease variability and identify suboptimal performance, thereby enhancing yield and ensuring high product quality. Fill out the form below to read the…
Integrated Features for Process Intensification
Biopharmaceutical companies are facing major challenges today, including rising drug manufacturing costs and lower production volumes related to unpredictable demands. To achieve the necessary reductions in both costs of goods (CoG) and infrastructure and operational costs, biopharmaceutical manufacturers integrate process intensification strategies into their production schemes. Novasep has embedded specific features into its BioSC™ platform to enable and accelerate process intensification (two key examples being viral inactivation steps management and in-line buffer preparation). BioSC Predict, Exclusive Simulation and Optimization Software:…
Improved Single-Use Technology for Online Turbidity Measurements
Turbidity is the relative clarity of a liquid as the result of suspended solids. Typically, turbidity measurements are taken by using a beam of light to detect the presence of particles and measuring the difference between the amount of light that is emitted from a source and the amount received by a detector. In bioprocess operations, liquid turbidity often is measured postfiltration to detect “break through,” or undesired materials coming from a filter, which provides an overall assessment of filter…
Benefits of Fluoropolymers for Frozen Storage of Bulk Drug Substance
Frozen storage of bulk drug substance (BDS) at temperatures at or below –70°C is common in the bioprocess industry. Storage at temperatures below –80°C can lead to spontaneous failure of typical BDS containers, particularly if container temperature is reduced rapidly (known as flash freezing). Most containers used to store BDS have glass-transition temperatures well above –196°C, and many containers structurally fail during rapid descent through glass transition. Even worse, such failures often are detected after a container is thawed, which…
Noncontact SONOFLOW®C0.55 Clamp-On Flow Meters and SONOCHECK®ABD06 Bubble Detectors
Noninvasive SONOFLOW clamp-on flow sensors and noninvasive SONOCHECK bubble detectors are designed for accurate and contamination-free upstream and downstream monitoring to fulfill regulatory goals within the process analytical technology (PAT) framework. Field-Proven Technology: SONOFLOW clamp-on flow sensors are designed for upstream and downstream bioprocess monitoring. These innovative sensors have integrated electronics that enable them to function without an external board or transmitter, providing a complete flow meter in a device the size of a small transducer. The systems can be…
Reduce Downstream Processing Costs for Monoclonal Antibodies: Switch to Tosoh’s Two-Step Platform
In this study, we showcase the benefits of Tosoh’s two-step process for the purification of monoclonal antibodies (MAbs) in comparison with the standard industrial process. Combining high-performance protein A capturing and a single polishing step on a salt-tolerant anion-exchange resin, we could reduce the downstream costs by 45% and increase production output by 58%. TOYOPEARL AF-rProtein A HC-650F is a high-capacity protein A resin for the purification of MAbs. This resin exhibits dynamic binding capacities (DBC) of 70 g/L at…
Who We Are
AbbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people, and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology, and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. Combining the focus of a biotechnology company with…
Giving You the Power for Clinical and Commercial Success
As a result of increased focus on customized medicine and continued emphasis on drug development for rare diseases, the biopharmaceutical industry continues to advance its methods for disease treatment by developing target-specific novel therapeutics. Formulations for these types of programs are complex and therefore present challenges in the design, scale-up, and manufacture of drug substances and drug products. Such challenges can delay getting critical products to patients. Contract development and manufacturing organizations (CDMOs) can help advance therapeutics and navigate production…
27 Years of Biologics Development and Manufacturing Experience
Avid Bioservices is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on development and manufacturing of biopharmaceutical drug substances derived from mammalian cell culture. Avid’s biologics development and manufacturing services include process development and current good manufacturing practice (CGMP) clinical and commercial drug substance manufacturing, bulk packaging, release and stability testing, and regulatory submissions support. For early stage programs, the company provides various process development activities, including upstream and downstream development and optimization, analytical methods development, testing, and…