Luina Bio, one of Australia’s most experienced biopharmaceutical contract development and manufacturing organizations, has announced plans to open a new good manufacturing practice (GMP) manufacturing suite. The expansion plans include a new small-scale GMP manufacturing suite that is scheduled to open in the fourth quarter of 2020. The suite will complete the company’s service offerings to customers that require small-scale GMP facilities. “This new suite will allow us to respond to those customers that need a smaller active dose for…
2020
eBook: Trends in Facility Design — In-House Manufacturing Considerations for Cell and Gene Therapy Production
Manufacturing and facility challenges facing cell and gene therapy companies are similar to but more complex than those encountered by companies that produce traditional biopharmaceuticals such as vaccines, monoclonal antibodies, and other therapeutic proteins. A single product can have multiple components, manufacturing of which may or may not be outsourced. Project timelines are short, production technologies are new and evolving, and clinical demands change rapidly. Increasing competition for contract manufacturing services requires reserving capacity far in advance, which in most…
New Data Analytics Tools Can Improve Bioprocess Workflows — If Applied Correctly
Biotherapeutics are a hot topic right now — and for good reason. But even before the COVID-19 pandemic, the biotherapeutics field, like every other manufacturing sector, was exploding with all the data that are being generated by recent innovations in equipment, systems, and processes. Advances in biomanufacturing analytics, analytical technology, and machine learning have tried to keep pace; however, such tools too often are misunderstood and applied suboptimally. Thus, many companies struggle with confusion and missed opportunities when they should…
Optimizing Cell Line Development for High-Quality Biologics
For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess…
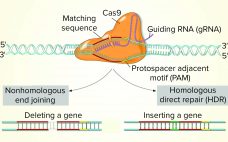
Use of CRISPR and Other Gene-Editing Tools in Cell Line Development and Engineering
While the role of biologics in treating human diseases has evolved dramatically over the past decade, so has genetic engineering. Rational genetic engineering to enhance biotherapeutic proteins has become a reality catalyzed by publication of the genome sequences of multiple Chinese hamster ovary (CHO) cell lines. Novel “designer” CHO cells modulate posttranslational modifications (PTMs) of recombinant proteins by genome editing, and it is now possible to knock-in or knock-out genes of yeast and mammalian cells precisely (within one DNA base…
Plant-Cell Cultures and Cell Lines for Recombinant Protein Expression
Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (1, 2). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity…
Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization
Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin G (IgG) product integrity in real time during a bioreactor run, especially…
From the Publisher: Thanks for the Ride!
By the time you read this, I will have officially called it a career at BioProcess International. Over 18 years — where did the time go? In many ways it feels wrong to be writing this — too soon, with too much still to do. But deep down, I know the timing is right. Professionally, it’s time to turn the reins over to the competent, experienced, and tireless staff you already know — either personally or through the pages of…
September 2020: From the Editor
I’m writing this from the height of our pandemic summer, and you’re reading it in the fall — in this strange year of time flying while seeming to stand still. We editors have worked from home for years, so our lives haven’t changed as much over the past few months as yours might have. Our Informa conference organizers, however, have seen their jobs transform radically. They’ve managed to pivot deftly toward virtual events, and I hope you’ve enjoyed some of…
Unraveling the Complexities of Technology Transfer
In the biopharmaceutical industry, technology transfer refers to transfer of any process, together with its documentation and professional expertise, between development and manufacture or between manufacturing sites (1). This operation is common in the biopharmaceutical industry for a number of structural reasons. They include the dichotomy between small, innovation-based drug companies and large ones able to conduct late-phase clinical development and endowed with manufacturing capacity; the high capital cost of biopharmaceutical plants, which makes contract manufacturing attractive; and the need…