As a full-service contract development and manufacturing organization (CDMO) specializing in gene therapy development, Yposkesi produces recombinant adenoassociated virus (AAV) and lentivirus (LV) vectors using adherent-based and suspension-adapted cell expression platforms. Alain Lamproye joined the company as chief executive officer (CEO) in January 2017, having served previously as president of the biopharmaceutical business unit of Novasep (2012–2017) and as CEO of Henogen, its subsidiary dedicated to gene therapy. He has held managerial positions in pharmaceutical operations at Merck Serono (including…
2020
Ask the Expert: Key Considerations for Cryogenic Preservation and T-Cell Viability
Cryopreservation provides critical protection for cell therapies by minimizing genetic changes. But cooling too slowly or quickly risks diminishing cell viability upon thaw. On 11 March 2020, Peter Kilbride (senior research scientist) and Julie Meneghel (cryobiologist), both of Cytiva (formerly GE Healthcare Life Sciences), discussed the importance of controlled-rate cryopreservation. Illustrating how mammalian cells change when frozen, Kilbride and Meneghel offered concrete cryopreservation strategies and identified temperatures at which it is safe to stop controlled cooling and transfer drug product…
Ask the Expert: A Robust, Stable Platform for Biologics Development
Sean Liour (vice president for project management at GenScript ProBio) delivered an Ask the Expert presentation on 18 March 2020 to explore the advantages of his company’s platform for cell-line development. Liour explained that successful biologic development hinges on robust host cell lines, capable expression vectors, and discriminating clone-screening systems. Overviewing relevant technologies and capabilities, Liour illustrated how his company’s ProCLD platform helps sponsors navigate cell-line development. Liour’s Presentation Researchers must weigh their options carefully when selecting a host cell…
A Healthy Biosimilars Market Promotes Innovation and Affordability
Innovative drug manufacturers require an opportunity to recoup capital and opportunity costs that they incurred to develop new medicines. Once patents have expired, competitors should be empowered to promote widespread affordability. An exclusivity period followed by a competitive market would promote the otherwise incompatible objectives of incenting innovation and promoting affordability. That careful balance exists for small-molecule medicines but not yet for biologics. The innovation side of the biologics market is working as intended, but a robust market for lower-cost…
eBook: Next-Generation Vaccines — COVID-19 Challenges, Opportunities, and Patent Questions
Resolving the COVID-19 pandemic depends on treatments, testing, and ultimately a widely disseminated vaccine against SARS-CoV-2. In recent decades, the biopharmaceutical industry has developed new approaches to vaccination using antigens, virus-like particles (VLPs), viral and bacterial vectors, and nucleic acids. Current events have placed those innovations at the front and center of public attention, offering many companies an opportunity to demonstrate their potential in an unprecedented way. Here, BPI’s senior technical editor describes the challenges that developers face in doing…
Navigating Technology Transfer
Technology transfer is a key milestone in the journey from discovery to full-scale good manufacturing practice (GMP)-compliant manufacturing. Navigating this step while preventing unforeseen issues that can create costly delays is supported best by combining knowledge of a given process with understanding of the technological capabilities. Different applications have different needs. Some challenges and goals are common to bioreactor processes for suspension and adherent cell culture for production of viral vectors, monoclonal antibodies (MAbs), other recombinant proteins, and vaccines. All…
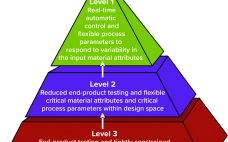
Developing Process Control Strategies for Continuous Bioprocesses
Process control enables biomanufacturers to ensure that operating parameters are within defined specifications. A control strategy should be established during early stages of process development while process and product performance are being defined using risk-based methods such as quality by design (QbD) and process analytical technologies (PATs). Confirming process control as an essential part of product development creates greater process knowledge and understanding and provides the first steps toward process optimization. By understanding how process performance relates to product quality,…
Industrialize Your Viral Vector Production in Adherent and Suspension Cell Cultures: Know the Pros and Cons
This educational podcast, “The Evolution of Culture Systems for Viral Vector Production: Advantages, Challenges and Cost Considerations,” recently published by Cell and Gene Therapy Insights, discusses in detail the pros and cons of viral vector production in adherent and suspension cell culture. This special report illustrates how Pall Biotech’s iCELLis 500+ bioreactors and Allegro STR bioreactors can bolster adherent and suspension culture, respectively, for viral vector production. Fill out the form below to read the complete report and learn more now.…
Are You Missing the Bigger Picture with Your AAV Analytics? Fast, Low-Volume Subvisible Particle Analysis for Characterizing Viral Vectors
Subvisible particle (SVP) analysis is a key indicator of stability and safety and is an essential parenteral drug quality metric. Yet assessment of SVPs is especially challenging in adenoassociated virus (AAV) vector formulations, in which limited precious sample is available. Traditional SVP methods consume large sample volumes, while dynamic light scattering (DLS), size-exclusion chromatography (SEC), and visual inspection can miss aggregates in the subvisible range entirely. This special report introduces backgrounded membrane imaging (BMI) technology as a solution for detection…
eBook: A Light-Chain Platform for Developing Bispecific Antibodies
Biopharmaceutical researchers are focusing on novel platforms to quickly develop bispecific antibody (BsAb) therapies for treating complex diseases such as cancer. BsAb therapies offer several advantages because they are designed to bind two unique epitopes. In this report, Bill Lundberg, president and chief executive officer at Merus, describes his company’s efficient approach to developing BsAbs using “common light chain” technology and other proprietary strategies. Lundberg also addresses the company’s approach to typical BsAb development and manufacturing challenges. Fill out the…