A key challenge for companies involved in drug development is to meet the highest standards of sterile design and reliability. In this context, magnetic mixers offers many advantages for aseptic stirring processes compared to mechanically sealed agitators. ZETA not only supplies magnetic agitators for new bioreactors, but also supports its customers through the entire retrofitting process, from feasibility studies at the beginning to full process qualification and validation at the end. “Combat the risk of batch contamination in bioreactors and…
2020
Reduce Downstream Processing Costs for MAbs By Switching to a Two-Step Platform
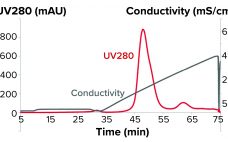
Downstream processing operations make up to 80% of the total costs for processing biotherapeutics. Given the current drive to reduce downstream costs, chromatographers and process engineers will need to streamline processes. Herein, we describe the benefits offered by using Tosoh’s two-step process for purifying monoclonal antibodies (MAbs) and compare that method with the standard industrial process. By combining high-performance protein A capture and a single polishing step on salt-tolerant anion-exchange resin, Tosoh’s approach can reduce downstream costs by 45% and…
Process Intensification of Viral-Based Vaccines: Where Are the Bottlenecks?
In the current coronavirus pandemic, the ability to scale up and produce viral-based vaccines (attenuated viral vaccines, inactivated viral vaccines, and viral vector vaccines) quickly and in large quantities has never before been more relevant. For viral-based vaccines that can be produced by adherent or suspension cell culture, process intensification — in which cell culture, for example, is optimized to produce higher viral titers using the same process equipment — offers a strategy to produce larger numbers of doses in…
Ask the Expert: Emerging Trends in Drug Assembly and Packaging
Thomas Gabriel (director of strategy and business development at Fujifilm Diosynth Biotechnologies, FDB) emphasized patient agency in his 15 April 2020 “Ask the Expert” presentation on innovations in finished-goods solutions. Facilitating self-administration significantly benefits patients with chronic conditions, and new delivery technologies are making drug products increasingly easy to handle and with increasingly accurate dosing. Gabriel explored how single-use autoinjectors and prefilled syringes, needle shields, on-body delivery systems, and digital monitors are improving drug-delivery safety and efficacy while enabling patients…
Ask the Expert: Improving Host-Cell Protein Detection By Enriching Mass Spectrometry Samples
Enzyme-linked immunosorbent assays (ELISAs) remain the industry standard for process monitoring and lot-release testing for host-cell proteins (HCPs), but researchers need orthogonal tests to confirm that an ELISA is fit for purpose. On 22 April 2020, Eric Bishop (vice president of research and development at Cygnus Technologies) delivered an “Ask the Expert” presentation about his company’s solution: mass spectrometry (MS) preceded by a novel Antibody Affinity Extraction (AAE) technique. Bishop explained that an AAE step can magnify a sample’s HCP…
Virtual Audits: A New Reality in the World of COVID-19
The novel coronavirus disease 2019 (COVID-19) pandemic has every industry seeking out ways to accomplish time-sensitive activities using a number of virtual approaches. This is certainly true in the biopharmaceutical sector, in which good manufacturing practice (GMP) audits are required to manufacture medicinal drug products for human use. Examples include supplier/vendor audits, mock inspections, and preapproval and prelicense inspections (PAIs and PLIs) conducted by sponsors and regulatory authorities. Auditors usually perform such activities on site and only sometimes remotely. In…
Managing Risk in Single-Use Systems Design and Implementation: A Shared Responsibility
Managing risk in single-use systems design and implementation is a shared responsibility. The ultimate responsibility for drug processes and products will always remain with manufacturers. However, implementation of single-use systems can shift responsibilities to suppliers within key areas, including design and sterilization, which must be clearly controlled and validated. This Special Report discusses how suppliers and manufacturers when working together can mitigate the risk of applying single-use systems in biopharmaceutical production from design through validation to point-of-use testing and operator…
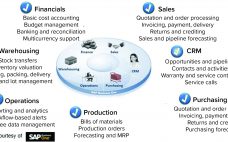
Challenges and Benefits of Networking Process Control Manufacturing Systems: Integration to Business Systems in Industry 4.0
Networking of manufacturing process control systems can lead to benefits of efficiency, increased productivity, and better facility use, leading to lower cost of goods (CoG). Furthermore, integration of manufacturing systems with manufacturing execution systems (MESs) upward to an enterprise resource planning (ERP) business system improves overall organizational efficiency. An ERP system enables optimization of intrafacility manufacturing resources. For multiple manufacturing facilities, it facilitates optimization across a company’s manufacturing network. Enabling Technologies and Systems At the enterprise resource planning level (Figure…
Designing the Right Strategy for Digital Transformation: How a Pragmatic Approach to Digital Transformation Can Help Biomanufacturers Adapt to a Challenging Future
Although the biopharmaceutical industry has enjoyed explosive growth over the past three decades, it still faces an assortment of challenges. Those include growing portfolio complexities, increased demand volatility, stringent regulatory requirements, increased pricing pressures, and growing technological complexities, all leading to severe pressure on profit margins. To overcome such pressures, biopharmaceutical operations need to become more reliable and agile, and they must realize efficiency gains in both manufacturing and supply chains. Digital transformation offers strong value opportunities, including a potential…
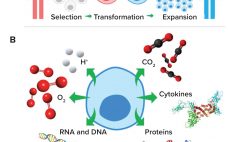
Fluorescent Nanosensors: Real-Time Biochemical Measurement for Cell and Gene Therapies
Cell and gene therapies are destined to transform the methods by which global healthcare challenges are approached and overcome (1). The US Food and Drug Administration is reviewing and approving an increasing number of cell and gene therapy products (2), and biopharmaceutical developers are dedicating immense resources to realizing the enormous potential of these therapeutics. Therefore, technologies that facilitate their effective and efficient manufacture will accelerate cell and gene therapies’ transition from medicines of the future to medicines of the…