As 2020 draws to a close, we can’t help looking back at this year in which the biopharmaceutical industry has received more attention than ever before — scientifically, politically, and otherwise. We editors always have been conscious of (and pretty good at) separating our work and home lives. As many of you have learned this year, that’s an important part of working from home, which we’ve been doing for over a decade. But in recent months, reading the news and…
November-December 2020
November–December 2020:
Don’t Be Forced to Accept a Bad Deal During the COVID-19 Crisis
Your boss’s boss just gave you the mandate to procure equipment, components, and raw materials for development and production of a new vaccine. Your goal is to get those as soon as possible — in addition to securing a massive volume for the future. You and your company are in the spotlight, and under significant pressure to deliver. You simply cannot fail. Yet many other players in your industry find themselves in the very same situation. Demand is exploding, and…
Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies, Part 2: Process Modeling and Analytics
Part 1 of this article focused on the first two sessions of a CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies,” which took place on 16–17 July 2018 in Gaithersburg, MD. Those sessions focused on upstream and downstream process technologies and strategies (1). Part 2 highlights the final two conference sessions on process modeling, control, and analytics. Process Modeling and Control The third conference session was “Modeling and Control Strategies.” Advancements in…
Accelerated Pathways for Authorization of Medicines in Europe and the United States
Before a medicinal product can be considered suitable for patients, it must go through laborious testing and cost-effectiveness analysis. In addition, all medicinal products must be authorized before they can be sold on the market and thus made available to patients (1). This is the case in the European Union (EU) and European Economic Area (EEA) countries as well as in the United States (US). Every year, a number of medicines receive marketing authorization. In their wake, however, several thousand…
Manufacturability Assessment: A Tool for Effective and Transparent Decision-Making and Efficient Process Development
Design for manufacturing (DfM, also known as design for manufacturability) is a common approach in engineering industries when complex, multistep production processes are developed and installed to manufacture products. Adherence to DfM approaches has been prevalent for decades in the automotive, aerospace, and electronics industries, among others (1–3). Recently, a generalized manufacturability-assessment tool with strategies to weigh different aspects of manufacturing has been proposed with numerous similarities to that described herein specific to the field of bioprocess development (4). Although…
Making Safe and Effective CAR T Cells: How Droplet Digital PCR Can Improve Their Quality Control
Chimeric antigen receptor (CAR) T cells first entered US clinics in 2017 (1), and this therapeutic modality holds tremendous potential as one of the most effective forms of personalized cancer care ever to reach patients. The revolutionary impact of CAR T-cell therapy comes from its ability to rewire our own immune defenses to kill cancer cells: It essentially modifies a patient’s naturally existing immune cells to boost their recognition and attack of cancer cells so that the person’s own immune system…
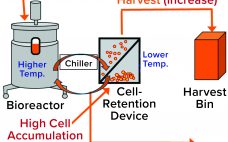
Product Quality Attribute Shifts in Perfusion Systems, Part 2: Elucidating Cellular Mechanisms
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (1). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits:…
Practical Considerations for Statistical Analyses in Continued Process Verification
Several statistical techniques can be used to assist in monitoring biopharmaceutical product quality attributes as part of continued process verification (CPV) activities. These include run charts, control charts, and capability analyses. Below, I provide an overview and recommendations on statistical strategies when developing a CPV program, considering the expected behavior of manufacturing results in the biopharmaceutical industry. Presence of Autocorrelated Data In a previous study, I highlighted the tendency for data to be positively autocorrelated (values are closely related to…
The Watershed Moment for ADCs Has Arrived — Now, CDMO Selection Is the Last Barrier
As far back as 10 years ago, antibody–drug conjugates (ADCs) were being talked about as the next big breakthrough in pharmaceuticals due to their highly targeted approach to therapies, especially in areas such as oncology. When Adcetris (brentuximab vedotin) became the second ADC approved by the US Food and Drug Administration (FDA) in 2011, it was predicted to be a watershed moment when the floodgates would open for more approvals and novel ADC approaches. Despite that possibility, the approval of…
Analytical Support for Biologics: A Conversation with Almac Sciences
Almac Sciences (a member of the Almac Group) recently expanded its suite of analytical solutions to include biologics testing. This follows a 2019 expansion of the company’s facility in Athlone, Ireland, where it provides a comprehensive range of flexible pharmaceutical testing services to support drug development programs adhering to industry regulations and good manufacturing practice (GMP) standards. “Biologics have gained huge traction in the past decade and are poised for stronger growth in the coming years with potential to significantly…