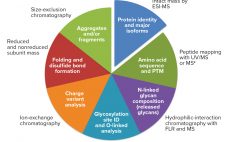
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (1). Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle…
2019
Embedded Particles in Single-Use Bags: Risk to Bag Integrity and Drug Product Purity, or Only a Cosmetic Defect?
When using single-use systems (SUS) to process biopharmaceuticals, preventing drug product contamination from extractables and leachables (E&Ls) and embedded particulate matter (gel particles) in the polymer films used to make bioprocess bags is critical. Using a pressure burst test to assess film integrity, Sartorius Stedim Biotech’s Klaus Wormuth and colleagues compared Flexboy and Flexsafe samples with gel-particle-free materials to assess their potential for contamination. The results showed that only large (2–4 mm2) gel particles affected the burst test results, concluding…
Ask the Expert: A New AEX Mixed-Mode Chromatography Resin for High Selectivity and Recovery
In an Ask-the-Expert webinar on 16 May 2019, Xuemei He (R&D manager of chromatography media chemistry at Bio-Rad Laboratories, Inc.) introduced the Nuvia aPrime 4A mixed-mode chromatography resin. Its macroporous particles exhibit good pressure-flow properties in addition to optimal ligand density and hydrophobicity. So this versatile resin offers effective separation of target from impurities in both flow-through and bind–elute modes, with good recovery of target proteins under gentle purification condition. He’s Presentation The resin is a hydrophobic anion exchanger with positively…
Ask the Expert: The MYCAP CCX System: Expand Outside the Hood
Charles Meadows (product manager at Sartorius Stedim Biotech) joined BPI for an Ask-the-Expert webinar on 13 June 2019 featuring the MYCAP CCX cell culture expansion system. He showed how a closed aseptic cell expansion process can decrease the rate of batch failure from contamination, in turn reducing waste and overhead costs. Meadows’s Presentation Contamination is an inherent risk of open cell culture expansion systems. Each passage from one flask to the next potentially exposes cellular material. A recent BioPlan Associates…
Ask the Expert: Efficient Technology Transfer: A CDMO Perspective
Effective transfer of technology from a sponsor facility to a contract development and manufacturing organization (CDMO) is essential to rapid biomanufacturing, especially for early and late-stage clinical supply. But the process is not as simple as “plug and play.” In an Ask-the-Expert webinar on 25 June 2019, Roy Sevilla (senior manager of upstream process development at Avid Bioservices) explained how his company orchestrates transfer operations with cross-functional teams. Strong, frequent collaboration with process sponsors ultimately mitigates risks associated with scale-up,…
Ask the Expert: Developability: Evaluating Specificity, Immunogenicity, Functionality, and Manufacturability for Lead Candidate Selection
Campbell Bunce (chief scientific officer at Abzena) joined BPI for an Ask-the-Expert webinar on 21 May 2019 to discuss his company’s approach to developability assessment, an increasingly popular way to identify leading drug candidates. Bunce explained what developability assessment is and how Abzena uses it to identify candidate liabilities and manage both clinical and financial risk during key stages of drug development. Bunce’s Presentation The high attrition rate of drug candidates from discovery to clinical proof-of-concept is well known. The…
Ask the Expert: GMP Flow Cytometry: Applications, Considerations, and Challenges
On 31 July 2019 Jamie Hall (project manager at Intertek Pharmaceutical Services) joined BPI for an “Ask the Expert” webinar to explore key applications and considerations for implementing flow cytometry (FC) methods in a GMP environment. This increasingly important technique can be applied to all stages of drug development in a range of applications, including cell-line identification, immunophenotyping, and cell-based potency assays. In a regulatory environment, such flexibility can present challenges. Hall explained how scientists can approach FC method development…
Toward Standardized Sample Collections: UK Government Seeks to Expand Cell and Gene Therapy Use
The SAMPLE program — a Standard Approach to ATMP Tissue collection — is intended to help build capacity for the UK National Health System (NHS) to expand the use of next-generation cell and gene therapies for both cancer and noncancer illnesses. Now it has been given the green light by Innovate UK (IUK), the United Kingdom’s technology strategy agency. An award through the Industrial Strategy Challenge Fund is supporting the program for standardizing how cell and gene samples are collected…
A Faster Solution to Hybridoma Screening
Monoclonal antibodies are a rapidly growing class of therapeutics and are used in multiple clinical indications. This highly competitive landscape necessitates the need for rapidly developing next generation antibodies against new challenging targets with improved mechanism of action and pharmacokinetics. Current antibody screening tools such as ELISA, only report on binding to one antigen target at a time; hence these assays are time consuming and require large amounts of target protein. This case study highlights how the Intellicyt® iQue3 system…
Gyrolab Immunoassays: Streamlining Workflows Through Automation
Immunoassays and other types of ligand-binding assays are widely used in the development of monoclonal antibody (mAb) therapeutics to measure antibody titers and support downstream purification processes by tracking impurities such as host cell proteins (HCPs) and leakage of protein A from affinity chromatography columns. While scaled down bioprocess development models and high-throughput testing generate large numbers of samples for analysis, immunoassays remain largely manual in many companies, resulting in high assay variability and low productivity. In contrast, automated immunoassays…