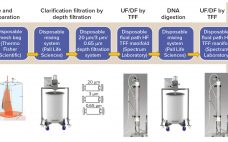
Autologous cell therapies are derived from a patient’s own stem cells, typically collected from bone marrow. Those cells are then cultured, expanded, and reinfused back into the patient. Unlike allogeneic cell therapies, this process is repeated for each dose and for one patient. The one-to-one process carries several challenges to commercialization, including high development costs, the need to control the risks of manual processing, and compliance with strict timelines. This eBook presents two perspectives on addressing these challenges. The first…
2019
From the Editor — September 2019
Our readers, authors, and advertisers often are surprised to discover that the magazine you hold in your hands (advertisements aside) represents primarily the work of three people. The BPI editors are multitaskers and jacks-of-all-trades by necessity. Every month we all seek out authors and work with them to develop their manuscripts . . . copyedit their texts and typeset the results . . . research, interview, and write our own articles . . . adapt and develop graphics . .…
A Challenging Future for Fetal Bovine Serum
Serum and other blood-derived products have been used widely in pharmaceutical research for many years. Use of these materials has contributed to many different advances in human and veterinary health, and they continue to have an important role in drug development. Fetal bovine serum (FBS) has had a specific role in the culture of mammalian cells for over 60 years. It is proven to be a useful tool for a broad spectrum of applications because it supports a large range…
Demystifying the Patent Search Process — Revisited
One of the first steps toward understanding how to patent an invention, invalidate someone else’s, or enter a new area of research is conducting a patent search. Knowing whether your invention is similar to those that already have been reported is critical before applying for a patent or beginning research. If your invention has been patented already or has been described or published anywhere in the world, then it is considered “prior art,” and your patent will be denied. Numerous…
Emerging Treatments for Spinal Cord Damage
Injuries to the spinal cord can cause permanent paralysis and even lead to death, with little or no hope for patients to regain lost function after such trauma has occurred. News of my spinal cord research first came to prominence in the 1980s with a front-page story that chronicles a presentation at the annual Society for Neuroscience meeting (1). That reported the first time that crushed peripheral nerves had been regenerated back into a patient’s spinal cord. Regeneration of spinal…
Biopharmaceutical Growth in China: Bioprocessing Challenges Are Creating CMO Opportunities
In the past decade, the world has seen rapid growth of more than 15% in China’s biopharmaceutical market (1). According to the second edition of BioPlan’s Advances in Biopharmaceutical Technology in China, the most populous country in the world — with the largest patient groups — has a growing economy with its GDP second only to that of the United States. Rapid urbanization and greater access to national healthcare insurance puts China in second place after the United States for…
Control of Protein A Column Loading During Continuous Antibody Production: A Technology Overview of Real-Time Titer Measurement Methods
During production of therapeutic antibodies, harvest titer is measured to monitor product mass loaded onto the protein A capture column. This prevents both column underloading (underusing expensive resin) and overloading (wasting product as flow-through (FT)) while allowing for column yield calculations. Batch production yields a single homogenous harvest pool, thus only one titer measurement (along with volume loaded) is sufficient to determine the mass loaded. During continuous production, however, cell-free harvest (permeate) continuously exits a perfusion reactor and loads a…
Advances and Challenges in Vaccine Development and Manufacture
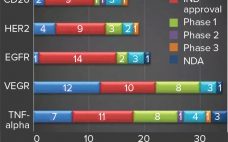
Scientists have made significant breakthroughs in bioprocess and analytical technologies for supporting vaccine development. Such technologies have helped vaccine manufacturers achieve consistent product purity and quality rapidly and cost effectively. Although interest in vaccine development and manufacture continues to increase because of the rapid growth of the global vaccine market, this area of the bioprocess industry remains challenging and complex. Here we review the current constraints and complexities in the vaccine industry, specifically related to product development and manufacture. We…
Formulation Development of Microbiome Therapeutics Localized in the Intestines
Scientists have discovered that microorganisms associated with the body play a critical role in human health (1). The intestinal microbiome is the collection of all combined genetic material of microorganisms in the intestines. An intestinal microbiota, a term frequently used interchangeably with microbiome, is the collection of such microorganisms in the intestine. With the volume of research into the human microbiome expanding, so too is the number of examples of its benefits to our health (1). Like diseases of the…
Time Is of the Essence: Optimizing Cell Line Development
Cell line development is a critical upstream step for the manufacture of monoclonal antibodies (MAbs). Cells must be engineered to produce a biologic of interest, and the right clone must be selected to deliver material for preclinical and clinical studies. A high-producing cell line is then needed to support clinical studies and, ultimately, commercialization of the therapeutic. Please fill out the form below to read more. Corresponding authors are Aurore Poles, Global Technical and Compliance Expert, and Guillaume Plane, Global…