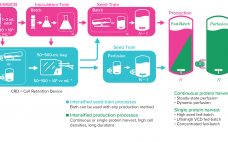
As the need for novel therapeutics increases, so does pressure on the biopharmaceutical industry to improve productivity, accelerate development, increase, and reduce costs — all while ensuring drug product quality. Upstream intensification strategies such as perfusion culture can address those challenges and achieve higher protein titers that can translate into higher throughput, improved flexibility, and compressed timelines. Successful implementation of perfusion culture or the transition to perfusion from fed-batch culture requires a different and strategic approach to media selection, not…
2019
Addressing Regulatory Requirements for Filter Integrity Testing
Filter integrity is a fundamental element of sterility assurance during production of biopharmaceutical and vaccine products. Integrity test results are a key foundation for drug lot release, so any external element that could affect their reliability must be viewed as a critical issue. But when should a filter integrity test be performed? This article highlights the Sartocheck 5 Plus filter integrity tester as a means to address regulatory requirements. Please fill out the form below to read the full article…
Forward Thinking — Evolving the Cell and Gene Therapy Industry: Modern CAR T-Cell Process Addresses Future Demand
It is an exciting time to be involved in the cell and gene therapy industry. We have come to a tipping point where we have shown that the science works. Now we need to industrialize and standardize it, so we can get these life-saving therapies to more patients. As an industry, we can learn from all the experience in the biologics space. With monoclonal antibodies (MAbs), we have transitioned from a manual process to a more standardized and automated set-up.…
Ask the Expert: Clarification of Lentiviral Vectors Using Scalable Filtration
Researchers traditionally have produced lentivirus (LV) in adherent cultures by transient transfection using media containing animal serum. The method is inexpensive for process development (PD) and early clinical trials, but it requires increased operator manipulations and costs during scale-up. Development scientist Adam McLeod delivered an Ask the Expert webinar on 27 August 2019 to illustrate how GE Healthcare Life Sciences at the Centre for Advanced Therapeutic Cell Technologies (CATCT) in Toronto, Canada, approaches lentiviral vector (LVV) manufacturing. McLeod explained that…
Production of Transient Lentiviral Vectors in HEK 293T Cells: Cultivation on Fibra-Cel Disks in a Single-Use, Packed-Bed, Stirred-Tank Bioreactor
Although demand for lentiviral vectors (LVs) for cell and gene therapy is increasing, the standard two-dimensional culture systems used to produce LVs present significant disadvantages. Current bottlenecks in LV production are caused mainly by such disadvantages. Switching to use of bioreactors can eliminate those problems because bioreactors offer the benefits of process automation, tight regulation of production conditions, and reduced labor input. The study reported herein was carried out by the group of David Parsons at the University of Adelaide.…
Single-Use Tubing Systems: Confidence Through Validation
Single-use systems (SUSs) are becoming increasingly common in bioprocessing operations because of their low capital requirements and validation costs. As this trend continues to develop, pharmaceutical manufacturers are asking SUS manufacturers to provide assurance that their products comply with current good manufacturing practices (CGMPs) and do not alter drug products by exceeding established operating ranges. Certification of product cleanliness has become common for manufacturers of final packaging components such as vials and stoppers, but rarely are tubing products certified to…
Ask the Expert: Automated, Closed-Loop, Inline Monitoring of CAR T Cells in a Production Process
Jan Van Hauwermeiren (vice president of sales and marketing at Ovizio Imaging Systems) joined BPI for an Ask the Expert webinar on 29 August 2019. He demonstrated how his company’s iLine F microscopy system combines sensitive optical capability with machine learning to monitor chimeric antigen receptor (CAR) T-cell expansion, transduction, and activation. The device captures holographic images of cells as they pass through a single-use conduit running between a microscope and bioreactor. Cells then funnel back into culture without additional…
Flow Monitoring in Continuous Processing and Single-Use Systems
Flow sensors placed at critical points in both upstream and downstream processes fulfill the regulatory goals of the process analytical technology (PAT) framework. PAT has been defined as a mechanism for design, analysis, and control of biotechnical and pharmaceutical manufacturing processes through measurement of critical process parameters (CPP). Constant flow monitoring can support its overall targets fundamentally to reduce production cycling time prevent rejection of batches enable real-time release increase automation and control improve energy and material use facilitate continuous…
Cell and Gene Therapy Leadership: Building Successful Teams
Cell and gene therapy (CGT) companies are opening the door to a paradigm shift in medicine. They’re rapidly developing some of the most exciting new therapeutics we’ve seen in decades, delivering meaningful results for patients and changing lives for the better. In the latest RSA Talent Equity report (1), we focus on the CGT sector, looking at the profile of leadership teams that bring the most value to shareholders in this exciting area. Growing Pressure in CGT CGT companies face…
Streamlining the Path to Clinical Manufacturing
Careful consideration of lot size is crucial for multiyear success of a cell therapy business. RoosterBio’s product design and acceleration business works with the company’s customers to help make plans that are appropriate for their stages of product and clinical development. We use the following major considerations in creating a sound multiyear strategy with intermediate milestones: Understand what the future looks like and work backward Use reasonable to conservative assumptions to estimate a range of needs Invest in the right…