Although the handling and preparation of cell culture media can seem routine, a number of risks are associated with such operations. Identification and mitigation of associated risks can help ensure consistency of performance, minimize likelihood of contamination, and protect employees while enabling greater efficiencies in upstream processes. Here we describe a number of strategies for reducing risks and streamlining media-related workflows. Simplifying Handling of Cell Culture Powders Media preparation typically is quite labor intensive and poses risks related to containment…
2019
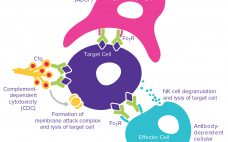
Mitigate Risk with Effector Function Characterization for Antibody Therapeutics
The complexities of biomanufacturing combined with heterogeneity introduced by cellular expression systems present significant challenges to assessing the quality of biologics such as monoclonal antibodies (MAbs). Information related to the critical quality attributes (CQAs) of MAb drug candidates is unknown during early phase drug development. It must be established empirically by physical, structural, and functional analyses as early as possible to accelerate development and mitigate risk through greater understanding of product characteristics. High-resolution analytical techniques are required to answer questions…
Launch of the First Vaccine Bioprocess Training Program: A Standardized but Flexible Course to Boost the Global Vaccine Industry
Based on the many forms that modern vaccines can take, their manufacturing is complicated. Unlike monoclonal antibodies (MAbs), vaccine manufacturers have no “template” platform to follow. Most vaccine producers develop their manufacturing processes from scratch, a prospect that can be challenging for small to mid-sized companies. Bioprocessing is the key challenge in vaccine manufacturing. Without a well-developed and understood process, a manufacturer will face serious challenges in commercial production: e.g., low yields, high costs, and difficulties in meeting quality standards.…
Ask the Expert: Scalable Purification Strategies for Viral Vectors and Vaccines
Viruses and virus-like particles (VLPs) raise unique challenges for downstream recovery and purification. In an Ask the Expert webinar on 19 September 2019, Mark Snyder (R&D applications manager of process chromatography at Bio-Rad Laboratories) discussed purification methods engineered to perform at manufacturing scales. Offering case studies featuring Nuvia resin and CHT Ceramic Hydroxyapatite chromatography support, Snyder emphasized that scalable media can help companies optimize downstream operations and address evolving manufacturing requirements. Snyder’s Presentation Tremendous growth in markets for vaccines and…
Ask the Expert: Risk-Based Approaches to Software Systems Compliance
Software risk management often seems synonymous with extra documentation. But during a 17 October 2019 Ask the Expert webinar, Martin Laferriere (director of information technology services at Avid Bioservices) explained how a thoughtful risk- assessment process at early project management stages can optimize software system validation efforts. Laferriere illustrated the benefits of developing strategic structures for documenting system use, hazards, and testing requirements. Such structures can help biomanufacturers clarify what records and functionalities they need to concentrate on to remain…
Ask the Expert: Rapid Virus Quantification in the Development and Manufacture of Emerging Therapies
The development and manufacture of emerging viral therapies is highly dependent upon accurate infective virus titers as well as total particle counts. But traditional quantification technologies have not kept pace with the needs of the viral vector market. Quantification methods based upon infectious particle counts can easily underestimate total particle counts. Indirect measurements such as enzyme-linked immunosorbent assays (ELISAs) and quantitative polymerase chain reaction (qPCR) are prone to overestimating the number of virus particles because they measure virus components to…
The Cell Therapy Industry Needs High-Quality Healthy-Donor Material
The cell and gene therapy (CGT) segment has grown tremendously over the past decade. And while the industry deals with a steep learning curve inherent to a rapidly developing field, problems must be solved, and solutions must be reduced to practice. One such problem, long understood by the industry but now thrust into the spotlight, is sample handling: proper collection, processing, preservation, storage, and transportation of cellular starting material. Suboptimal techniques for such logistics have been shown not only to…
Bioprocess Intensification – Fast, Flexible, and Efficient Solutions
Propelled by single-use systems (SUSs), biopharmaceutical companies are approaching the ideal of continuous bioprocessing. In addition to improving process integrity and decreasing production costs, SUSs have enabled exciting ways to configure, operate, and evaluate manufacturing steps. Sensitive process analytical technologies (PATs) and discriminating data analysis platforms are supplementing those developments, helping process engineers and operators to study and modify workflows in unprecedented ways. The goal now is to intensify: to apply increasingly nuanced process knowledge and growing technological capability in…
eBook: Addressing Production Complexities — Strategies for Working with Difficult and Susceptible Proteins
All proteins are complex — but some are more complex than others, particularly when it comes to recombinant protein expression and production in commercial quantities. What works in a research laboratory to make a milligram of pure protein for study won’t necessarily work on a manufacturing floor to make kilogram batches for drug-product formulation. An increasing number of technological options are available, however, from a simple switch in expression host or adding folding steps in downstream processing to special genetic…
BioProcess International 2019 Event Report
The 2019 BioProcess International Conference and Exhibition, held in Boston, MA from 9–12 September, was a testament to the rapid expansion of the biopharmaceutical industry. Nearly 150 speakers chronicled recent developments and continuing challenges in upstream production, downstream processing, drug product manufacturing, and emerging therapies production. And with more than 150 poster presentations and over 200 companies participating, the BPI exhibit hall never better embodied the industry’s efforts to support increasingly diverse but related audiences. In this event report, BioProcess…