The vast majority of monoclonal antibody (MAb) production processes are based on fed-batch Chinese hamster ovary (CHO) cell culture and protein A affinity column chromatography capture. Increasing cost-consciousness — among innovator companies as well as biosimilar makers — has many companies looking “beyond the platform” for less expensive alternatives that may provide better results. Here the BPI editors review some state-of-the-art alternatives in upstream and downstream MAb drug substance bioprocessing as well as drug-product manufacturing. The current “gold standard” platform…
2019
Emerging Tools for Exosome Purification and In-Process Monitoring
This eBook introduces new analytical approaches that enable in-line chromatographic detection of exosomes. One approach can discriminate extracellular vesicles from nonvesicle contaminants, and one potentially can discriminate exosomes from other vesicles. Examples illustrate how they enable development of more effective and better documented purification methods. The special qualifications of monolithic chromatography media for exosome purification are discussed. New process tools designed to accommodate some of the special challenges of exosome purification are introduced. Exosomes represent one of several species of…
Introduction: Drug Product Discussions
Quality by design (QbD), risk management, and new technologies are shaping biologics formulation work in the 21st century. We saw much evidence of this at the BioProcess International Conference and Exhibition in Boston last fall, where a wide range of talks filled the Drug Product, Fill–Finish, and Formulations track during the week after Labor Day. Dingjiang Liu (Regeneron) offered a high-level discussion from the BioPhorum Development Group (BPDG) on “An Intercompany Perspective on Biopharmaceutical Product Robustness Studies.” Such studies ensure…
Analytical Strategies for Fixed-Dose Coformulated Protein Therapeutics
Coformulation of two or more proteins in a single formulation is an emerging approach to delivering multiple biotherapeutics that previously have been administered in sequence. This approach brings multiple benefits to all stakeholders. Foremost for patients, the primary benefits are combined therapeutic effects and improved convenience (e.g., fewer administration events). Healthcare providers see logistical benefits and decreased risk of medical errors. Additionally, coformulations also simplify manufacturing logistics, reduce costs of packaging and distribution, and provide new opportunities for product portfolio…
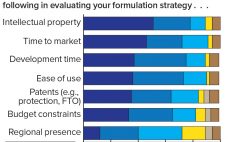
Early Stage Development of Advanced Formulations in the Drug Development Process Provides Competitive Advantages: Survey Predicts That Drug Product Formulation Recognition and Budgets Will Increase Significantly
New antibody formats and aggregate-prone, subcutaneously administered protein therapeutics present biopharmaceutical companies with major challenges regarding protein stability and aggregation. At the same time, protein stability often is not given enough attention in early stages of development. Protein aggregation reduces drug activity so that increasing doses are needed to achieve the same desired effect. Even worse, protein aggregates can induce immunogenecity that endangers patients and compromises product approval. A market study presented for the first time at the Bio-Europe 2018…
BioProcess International Europe 2019: Event Planner
BioProcess International Europe has earned its reputation as the leading and largest European bioprocessing industry event. It is a key time each year when the industry connects to share new ideas and innovations across all phases of bioprocessing: from cell line development through upstream and downstream, with bioprocess analytics, viral safety, continuous manufacturing, and vaccines. This year’s meeting on 2–4 April 2019 at the Messe Wien Exhibition Congress Center in Vienna, Austria will attract more than 900 scientists, engineers, and…
March From the Editor
The merging of Informa’s KNect365 division with pharmaceutical events operated by UBM brings another opportunity for BPI’s successful “BPI Theater” program: the “BioLIVE” theater taking place at CPhI. Our first foray into the theaters — our own speaker/roundtable sessions held in the exhibit halls of large events — was at a BIO International Convention some years ago. Through our relationship with the Biotechnology Innovation Organization, that came about to bring more technical content into the big event. As BIO grew…
Frameworks and Strategies for Commercialization Success in the Biopharmaceutical Ecosystem
The “biopharmaceutical ecosystem” is a multibillion-dollar industry that encompasses large and small drug companies; ancillary providers of services, technologies, equipment, and infrastructure support; and tertiary groups that provide policy direction, regulatory standards, incubator space, and more. This ecosystem is vast and dynamic, evolving constantly as new ideas take hold to push the industry in new directions and present new opportunities for innovation and commercialization. However, many companies operating within this ecosystem struggle to understand their development and commercialization strategies, and…
China Can Be Ignored No Longer: Long-Term Biopharmaceutical Opportunities Based on Near-Term Demonstrated Growth
China has long served as “the world’s factory,” but many experts in the biopharmaceutical industry have assumed that making consumer electronics, clothing, and toys for a global consumer base does not translate well to making complex biologics on the global stage. However, according to a newly released report, the biopharmaceutical market in China reached a value over US$9 billion in 2018, with the domestic monoclonal antibody (MAb) market there making up a very large percentage of that (1). Much of…
Recommended Practices for Assuring Integrity of Single-Use Systems
The increasing uptake of single-use technologies (SUTs) in critical current good manufacturing practice (CGMP) processes and applications has made their integrity a critical quality attribute (CQA) for both suppliers and end users of such systems. Current regulations focus on final packaging, however, without taking into account the unique aspects of assemblies used in bioproduction. Ongoing initiatives include revision of PDA TR 27 (1) and creation of A STM workstreams (2, 3) to propose good practices for the integrity of single-use…