In the history of regenerative medicine, 2017 was a critical year. With approvals for Kymriah (tisagenlecleucel) from Novartis AG, Yescarta (axicabtagene ciloleucel) from Kite Pharma (a Gilead company), and Luxturna (voretigene neparvovec-rzyl) from Spark Therapeutics, cell and gene therapies finally made their mark on the regulatory landscape. Then in 2018, those products began both treating patients and bringing in revenues for their sponsor companies. “Patients are being treated, and biotechnology and pharmaceutical companies are being paid for treating them,” said…
2019
The Role of Single-Use Polymeric Solutions in Enabling Cell and Gene Therapy Production: Part 1: Introduction and Manufacturing Process
by Bio-Process Systems Alliance Cell and Gene Therapy Committee The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plastic-equipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development…
Innovation Leadership in Drug Development
A “sea change” in the biotechnology and pharmaceutical industries is leading established players to recruit a new type of drug-development leader. Disruptive innovators such as LG Chem Life Sciences, Google, and NestlĂ© are challenging established life-science companies to be nimbler, more creative, and more adept at applying new and emerging technologies. To spur creativity and entrepreneurship in research and development, smaller companies and Big Pharma corporations alike are recruiting leaders from different fields both inside and outside the life sciences.…
A Perspective on GMPs for Cellular Therapy Commercialization
Cellular therapies can be classified by therapeutic indication, by cell types, and by whether cells are taken from and administered to the same individual (autologous) or derived from healthy donors (allogeneic). Regulatory classification of cellular therapies differentiates among minimally manipulated cells for homologous use, transplants or transfusions, and cells that are more than minimally manipulated and regulated as medicines. Medical cellular therapies must meet quality, safety, and efficacy standards to obtain marketing authorization (1–8). Such therapies can be subdivided into…
Scientific and Technological Advancements in Applications of Single-Use Technology: A Conference Report
Single-use technology (SUT) has been used increasingly both in clinical and commercial biomanufacturing (1). Proven major advantages include relatively low capital investment, elimination of batch-to-batch cross contamination and reuse cleaning validation efforts, flexibility in manufacturing, and shortened product lifecycles. However, some challenges and barriers to implementation remain: Consumables costs are increasing. Specific regulatory guidance is lacking, as is component interchangeability and standardization. And few if any leak-proof components/systems are available. International groups and associations focused on setting best practices and…
Cell and Gene Therapy Data Management: Solutions to Address Complex Challenges
At the Phacilitate Leaders World and World Stem Cell Summit 2019, held 22–25 January, Steve Goodman (head of drug product manufacturing at bluebird bio) and Robert Di Scipio (CEO of Skyland Analytics) shared the podium to address what the product and process data-management ecosystem looks like for cell and gene therapy (CGT) development and manufacturing. A starting point for their presentation was that CGT development presents significant data challenges: capturing and analyzing product development and manufacturing data, tracking the collection,…
2019 BioProcess International West Event Report
At the BPI West conference in Santa Clara, CA, common themes wove throughout multiple tracks specific to process stages and levels of regulatory scrutiny. The BPI West program brings upstream and downstream experts together into the same sessions based on whether they share early or late-stage concerns. Even though special tracks addressed viral safety and technological advancements this past March, and preconference symposia focused on specific topics, a significant amount of session overlap throughout the week ensured that attendees could…
Visible Particulate Matter in Single-Use Bags: From Measurement to Prevention
Parenteral pharmaceuticals must be “essentially free” from visible particulate matter (1). In the production of biopharmaceuticals with single-use systems (SUS), biocompatibility requires controlling interactions between drug substances/products and SUS surfaces to ensure drug product quality and patient safety with regard to extractables/leachables and particulate matter. Any particulate matter stuck to fluid-contacting surfaces of process components could wash off and contaminate process fluids. Depending on system configuration, a final drug product could be at risk for particulate matter from SUS. Risk…
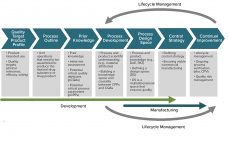
Is the QbD Toolbox Ready for Cell and Gene Therapies? Integrating Patient Outcomes into Manufacturing Cell and Gene Therapy Bioproducts
In their lifecycle development and manufacturing models, biotechnology products and the biopharmaceutical industry have been founded on principles originating from the pharmaceutical small-molecule industry. Such principles define clinical programs that establish risk benefits of a dosage and its delivery system on healthy individuals and patients. A company then develops a process to manufacture that product consistently over several years. Product quality attributes set through manufacturing controls are expected to ensure patient outcomes in terms of safety and efficacy and deliver…
Competing for Talent: Make Your Biotech Workplace a Powerful Asset
Talent is the lifeblood of pharmaceutical innovation. But there’s more to winning top talent than simply recognizing the value of our “human resources.” It also takes putting that value into action by giving people a workplace that inspires and even delights. Today, forward-looking industry leaders are seeing competitive advantage in rethinking workplace strategy and the role it plays in attracting and retaining talent, according to a 2018 report (1). Once considered to be merely a passive background for discovery, a…