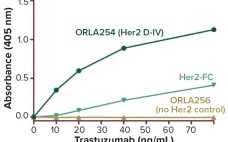
Speed to clinic testing — and then speed to market — are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial “design of experiment” (DoE) analysis…
2019
May 2019 From the Editor
One thing that differentiates Baby Boomers, GenXers, Millennials, and Generation Y is their communication preferences. Who is most likely to answer a call or email, default to texting, or use a given social-media platform? Newer generations of bioprocess professionals are building on the legacies of this industry, and their fresh perspectives are driving innovation. BPI strives to help share that work with as large and relevant an audience as possible — in a range of formats that includes something for…
Why Conducting Marketing Due Diligence Early in Product Development Is Important
To be successful, a company needs two main ingredients: good science and good business/ marketing. Without good science, a product won’t work, and without a good marketing strategy, a product won’t sell. Two important questions should be addressed: When should marketing groups be involved in product development, and how important is that? The answer to the first is as early as basic research. Why? After a product is launched, a biomanufacturer doesn’t want to discover that its product applies only…
Breaking Through the Noise: An Approach to Differentiating Your Business
When multiple businesses sell the same type of item, why do customers buy from one supplier rather than another? How are vendors able to break through the “white noise” of an industry to stand apart and get noticed? The current bioprocessing and cell therapy vendor markets, about 50,000 vendors are selling to biomanufacturers, universities, and research institutions (www.bioz.com). How do those suppliers get noticed? Market-leading suppliers have established brands that allow them to cross-sell, up-sell, and engage in deep selling.…
China Looks Inward and Outward: Investments in US and EU Biopharmaceutical Companies Are on the Rise
The growth momentum of China’s biopharmaceutical industry continues, with the China Industry Research Institute projecting that the country’s biological therapeutics market could reach 300 billion Chinese yuan (~US$50 billion) in 2019. According to the second edition of our report on advances in Chinese biopharmaceutical technology, this robust growth is boosted by continuing investment into the sector (1). A clear strategic indicator of the country’s intentions in biotherapeutics and biologics has been government investment in bioindustrial hubs, which by next year…
Biologic Labels and Induced Patent Infringement: A Perspective on Evolving US Law
The mechanism for proving patent infringement is changing for developers of both branded and follow-on biologics (either biosimilar or interchangeable). Here we examine how drug labeling can establish infringement, thus affecting follow-on manufacturers accused of inducing others to infringe patents on methods of treating medical conditions. Because precedent is paramount in the US legal system, judges look to small-molecule case history to help them understand alleged infringement by follow-on biologics. The classic approach to induced infringement of generic small-molecule drugs…
Modeling Virus Clearance: Use of a Noninfectious Surrogate of Mouse Minute Virus As a Tool for Evaluating an Anion-Exchange Chromatography Method
Viral safety is a critical focus during biopharmaceutical manufacturing (1–5). Although well-characterized mammalian cells such as the Chinese hamster ovary (CHO) line have been used for decades, both endogenous expression of retroviral-like particles and exogenous contamination events from viruses warrant continued vigilance (6, 7). International regulatory agencies require biomanufacturers to validate the “viral clearance” efficacy of their downstream manufacturing process steps before resulting products can be awarded clinical trial or commercial approval (8–10). Currently, viral clearance testing is based on…
The Role of Single-Use Polymeric Solutions in Enabling Cell and Gene Therapy Production –
Part 2: Regulatory Overview
The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plasticequipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and…
The Proper Use of Extractables Data: Aspects Beyond Extractables Measurement
This webcast features: Dr. Armin Hauk, Lead Scientist, and Jean-Marc Cappia, Head of Segment Marketing Vaccines, at Sartorius Stedim Biotech The implementation and use of single-use system (SUS) in biopharmaceutical production is rapidly increasing and directly correlated with higher demands on Extractables and Leachables (E&L) information. Sartorius as a leader in SUS technology can look back on +20 years of experiences in the E&L area. This knowledge together with our current research initiatives enables us to progress and go beyond…
Risk Management of Biopharmaceutical Operations: End-to-End and Over Lifecycle
Biopharmaceutical manufacturing processes that were developed before the implementation of quality by design (QbD) typically use control strategies that are not founded on a formal understanding of criticality. Thus, manufacturers of “legacy” products lack the understanding of critical process parameters (CPPs) and critical quality attributes (CQAs). Introducing such elements to a legacy biologic drug product filing aligns fully with expectations described in the ICH Q12 guideline (e.g., increased process understanding and better risk mitigation strategies) (1). Here we discuss how…