In biopharmaceutical manufacturing, sterility, speed, and equipment ease of use are essential factors. CPC’s AseptiQuik® connectors are specially engineered to provide all three. They create quick and easy sterile connections, even in nonsterile environments. They also allow you to transfer media easily with less risk of operator error than other connectors. All AseptiQuik connectors assemble in an easy-to-use three-step process and come in a wide range of size options and genderless connections. Fill out the form below to read the…
2019
Efficient Single-Use Method for Midstream Cell Removal Using Filter Aid
Cells and cell debris are removed between fermentation (upstream) and product cleaning (downstream) during a process called midstream. Midstream processes often are performed by combining several unit operations (1). A highly efficient method is cake filtration, which can be conducted using a FILTRODISC™ BIO SD depth filter. Here, I describe the cleaning of fermentation broths using cake filtration. This technology can maximize product yield and economic efficiency. With increasing particle loads (>108 cells/mL), standard technologies (e.g., centrifugation, separation, and membrane-…
CYLINDRAFlow™ Manifolds: A New Offer That Simplifies Processing Applications
Nordson MEDICAL has launched a new manifold that makes assembly and operation simpler compared with products currently used in the biopharmaceutical industry. This patent-pending product was announced at ACHEMA in Frankfurt, Germany, in 2018. Simplicity is the main benefit of the design and user interface built into CYLINDRAFlow™ manifolds. There is a one-to-one relationship between the inlet port and the five available outlet ports. The large, easy-to-use knob with clearly marked arrows provides a visual indication of which outlet port…
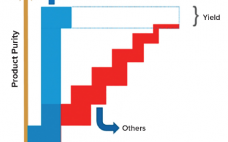
Control Strategy in Continuous and Intensified Bioprocessing
Pharmaceutical companies take several factors into account when deciding whether to switch from established batch processes to better performing ones, including continuous processing. Such issues include the need to cut cost of goods (CoG), improve product quality consistency, increase process robustness, prevent human error by implementing automation, and so on. However, some challenges remain for some companies integrating these new ways of processing into a current good manufacturing practice (CGMP) environment. One issue is the adaptation of a quality management…
Tools For Early-Stage Purification Development of Filtration Operations
Detailed early-stage development of filtration unit operations is critical to achieving a robust and reliable full-scale process. Despite the importance of this type of development work, fully evaluating a specific unit operation often can be difficult because of time constraints and a lack of material available for experimentation. As a result, a laboratory must have the proper equipment required to conduct efficient, high-throughput experiments. PendoTECH® has developed the ideal tools to address these types of challenges and enable laboratories to…
Development of Affinity Chromatography Adsorbents for Bioseparations
Affinity chromatography has a long history of use in biopharmaceutical manufacturing and is a powerful tool in making industrial processes more efficient by reducing the number of steps required to achieve the desired target purity and yield of a final product. It is common, however, for teams to consider affinity chromatography once all other options have been considered, evaluated, and discarded. Such an approach usually translates into a need to develop affinity adsorbents for new targets quickly, efficiently, and economically.…
Combining Novel Jetting Manufacturing Technology with a New, Alkaline-Stable Protein A Resin
Praesto® Jetted A50 is the first protein A resin to meet the future demands in monoclonal antibody (MAb) processing today. It delivers enhanced performance characteristics through Purolite’s patented manufacturing technology: Jetting. This continuous manufacturing process — combined with a novel, alkaline-stable continuous manufacturing process with a novel, alkaline-stable protein A ligand called NGL Impact A™ (supplied by Repligen Corporation) — results in the only bioprocess-scale agarose available with a uniform particle-size distribution. Compared with other resins, Praesto® Jetted A50 resin…
Benefits of Fluoropolymers for Bioprocessing
Fluoropolymers play a critical role in the storage, freezing, and shipping of critical bioprocess fluids. Not all fluoropolymers are ideally suited for all applications, so it is important to understand the characteristics of each and the benefit of using one over another for your specific application. Most Common Fluoropolymer Materials Fluoropolymers have more commonalities than differences. Such characteristics include: Extremely high purity compared with other plastics used in bioprocessing, including very low or no measurable leachables or extractables Inert and…
Sonoflow CO.55 Clamp-On Flow and Sonocheck ABD Bubble Sensors
With their integrated electronics board, Sonoflow and Sonocheck sensors offer the smallest equipment footprint solution on the market. By using SONOTEC’s reliable and reusable sensors, you can improve process stability, achieve easy data transfer, and save costs associated with disposables. With the aim of implementing flexible production and minimizing contamination risk, a number of applications in the biotechnology industry need accurate flow measurement and bubble detection. Fill out the form below to read the complete technology review now.
Ajinomoto Bio-Pharma Services
Ajinomoto Bio-Pharma Services is a fully integrated contract development and manufacturing organization (CDMO), with sites in Wetteren and Balen (Belgium) and San Diego (California, USA). We provide comprehensive process development services, current good manufacturing practice (CGMP) manufacturing, and drug-product fill–finish services of small-molecule and biologic-active pharmaceutical ingredients (APIs) and intermediates. Fill out the form below to read this capabilities review now.