One of our annual tasks as a publication staff is to develop next year’s editorial calendar. How well are we anticipating new directions in research, development, and manufacturing? Are we including interesting and forward-thinking topics — or relying too heavily on frequent, overintroduced themes? We have seen an overall generational change in conference attendance and our reading audience. So we also ask ourselves whether we are correctly assessing information needs and providing accessible levels of detail. It’s not a perfect…
October 2019
From Supplying Components to Providing Total Solutions: Overviewing Supplier Side Capabilities
Only a thin line now separates biopharmaceutical manufacturers and suppliers because the latter are increasingly becoming the process knowledge owners in the biopharmaceutical industry. As a result, suppliers are racing to become the most efficient “total solutions” provider. In the 1990s, leading players in the industry such as Pall, Millipore, and Sartorius all supplied membrane filters for upstream and, to some extent, downstream processes with their crossflow and final filtration offerings. Pharmacia (which became GE Healthcare) was the major force…
Bioprinting Capabilities and Futures
Bioprinting has advanced rapidly through engineering step changes in the use of three-dimensional (3D) printing. With these developments, living cells can be positioned layer by layer to produce functional tissue structures. Key attributes of this emerging technology are its high scalability and modularity, which enable automated and repeatable manufacture of a wide variety of tissues. These high-throughput biofabrication capabilities equip companies with tools to develop 3D-printed tissues for broad applications, from in vitro drug testing models to therapeutic tissue implants,…
The First Quantitative Industry Assessment of Single-Use System (SUS) Reliability: Raising the Bar for BPSA’s Value to Industry
Over the past 24 months, the leadership of the Bio-Process Systems Alliance (BPSA) has initiated a pilot program to demonstrate the joint feasibility of collecting and sharing pertinent industry business data. We began with “quality-complaint data” centered on the premise that product complaints are tightly associated with product-quality defects in single-use systems (SUSs). This approach can be viewed as “risk assessment 101.” BPSA’s Representation of the Single-Use Industry BPSA is an industry organization primarily comprising drug manufacturers, single-use system suppliers,…
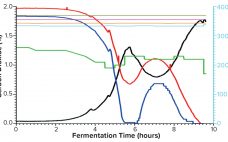
Comparative Study of Single-Use and Reusable Fermentors: Production of Recombinant Proteins Through Bacterial Fermentation
Single-use bioreactors have become widely accepted and well established for cell culture applications in the biopharmaceutical industry for over a decade (1). Abbott Diagnostics has moved into this technology already for commercial production of some biologic molecules. However, single-use systems (SUSs) are rarely available for microbial applications, mostly because of the technical challenge in designing cost-effective SUSs that can meet high oxygen transfer needs and remove excessive heat generated during fermentation. Thus, an important part of our biologics manufacturing —…
Viral Nanofilter Integrity: Using Variable-Pathlength UV-Vis Spectroscopy for the Gold Nanoparticle Test
Viral filtration (VF) using nanofilters removes endogenous and/or adventitious viruses from biologic drug-substance manufacturing processes (1). The gold particle test (GPT) is performed as part of postuse integrity testing — to complement postuse leakage testing — for cellulose filters such as Planova 20N filters from Asahi Kasei Corporation. First, a proprietary gold-colloid solution matched to the filter type (e.g., 20N) is filtered through the test article. That filter’s pore-size distribution can be assessed using spectrophotometric absorbance readings of the integrity-test…
Adenovirus Downstream Process Intensification: Implementation of a Membrane Adsorber
Historically, companies developing vaccines have used attenuated pathogens, inactivated infectious agents, or antigenic constituents purified from pathogenic sources. In the past 20 years, technological advances such as recombination and viral vectors, have enabled development of vaccines against diseases with previously no available treatments (1). Viral vectors have become one of the most rapidly evolving and promising fields in vaccinology and regenerative medicine. In addition to preventing infectious disease, they have a broad range of potential applications, including treatment of hereditary…
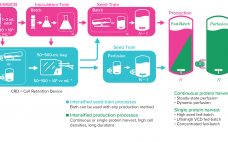
The Critical Role of Media in Intensified Upstream Processes
As the need for novel therapeutics increases, so does pressure on the biopharmaceutical industry to improve productivity, accelerate development, increase, and reduce costs — all while ensuring drug product quality. Upstream intensification strategies such as perfusion culture can address those challenges and achieve higher protein titers that can translate into higher throughput, improved flexibility, and compressed timelines. Successful implementation of perfusion culture or the transition to perfusion from fed-batch culture requires a different and strategic approach to media selection, not…
Addressing Regulatory Requirements for Filter Integrity Testing
Filter integrity is a fundamental element of sterility assurance during production of biopharmaceutical and vaccine products. Integrity test results are a key foundation for drug lot release, so any external element that could affect their reliability must be viewed as a critical issue. But when should a filter integrity test be performed? This article highlights the Sartocheck 5 Plus filter integrity tester as a means to address regulatory requirements. Please fill out the form below to read the full article…
Forward Thinking — Evolving the Cell and Gene Therapy Industry: Modern CAR T-Cell Process Addresses Future Demand
It is an exciting time to be involved in the cell and gene therapy industry. We have come to a tipping point where we have shown that the science works. Now we need to industrialize and standardize it, so we can get these life-saving therapies to more patients. As an industry, we can learn from all the experience in the biologics space. With monoclonal antibodies (MAbs), we have transitioned from a manual process to a more standardized and automated set-up.…