“We are witnessing one of the most significant paradigm changes in oncology drug development, with some new types of immunooncology compounds inducing unprecedented increases in survival in certain solid and liquid tumor indications.” —Jagath Reddy Junutula (Cellerant Therapeutics) and Hans-Peter Gerber (Pfizer) (1) An antibody–drug conjugate (ADC) for cancer treatment needs four things to succeed clinically: It needs to target the right antigen using the right antibody, with the most powerful cytotoxin attached by best linker option. These features combine…
May 2019 Featured Report
Antibody–Drug Conjugate News: From BioProcess Insider
The following news items have appeared on the BioProcess Insider site over the past year. Together they indicate the direction in which the ADC sector is moving. BEYOND ADCETRIS — SEATTLE GENETICS AIMS FOR BIG BIOPHARMA STATUS 30 April 2018: Seattle Genetics is building toward a bigger future. For the first quarter 2018, the company’s total revenues grew to US$141 million (€116 million) compared with $109 million in the same period last year. This was attributed to a 36% increase…
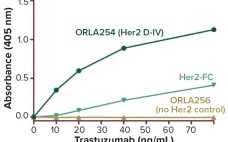
Analytical Tools to Improve Decision-Making During Product Development
Speed to clinic testing — and then speed to market — are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial “design of experiment” (DoE) analysis…