Avid Bioservices is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on development and manufacturing of biopharmaceutical products derived from mammalian cell culture. Avid provides process development and current good manufacturing process (CGMP) clinical and commercial product manufacturing and offers expertise supporting analytical development, qualification, and validation activities. Additional service offerings include cell line optimization, cell culture and feed optimization, product characterization, and stability testing. Avid has 26 years of experience producing a comprehensive range of proteins, including…
Industry Innovators 2019-2020
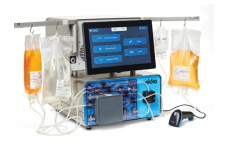
ekko™ Acoustic Cell Processing System
The ekko™ system is a good manufacturing practice (GMP)–capable platform technology for cell and gene therapy manufacturing. Based on the innovative application of acoustics, the ekko system can process cells gently and efficiently in ways that traditional mechanical and filtration methods cannot. With a wide range of operating conditions, the ekko system can be used to concentrate and wash cellular material in multiple unit operations, from early research through to GMP production. Fill out the form below to read the…
Freezing of T Cells Using the VIA™ Freeze System
Autologous T-cell therapies no longer are limited to small, single-site trials. National and international demand is growing, bringing with it the demand for rapid scaling of manufacturing. But there is little margin for error, because any variables in an end-product could result in a potentially hazardous change to a therapy’s potency. Cryopreservation is a critical tool for quality control and transport of cells. Liquid nitrogen (LN2)–based controlled-rate freezers have long been used to freeze clinical-grade T cells. Cells are frozen…
Process for Production of Oncolytic Adenovirus
Oncolytic viruses constitute a new promising therapeutic approach for treating cancer. These viruses selectively replicate in tumor cells and effectively kill those cells without harming normal cells. Selectively engineered, the viruses do not only destruct target tumor cells, but they also stimulate the host’s antitumor immune response. Adenoviruses are extensively characterized, well-studied viral vectors that infect both dividing and nondividing cells without the risk of integration into the host genome. Generally causing a mild nature of disease, adenoviruses are considered…
The Next Milestone in Cell and Gene Therapy
The field of cell and gene therapy is transforming the way patients who are diagnosed with cancers or genetic diseases can be treated. Today, the cost of producing these therapies still represents a major hurdle on the way to the commercialization. New technologies are needed that enable robust and cost-efficient manufacturing and yield replicable high-quality medicines. Although the therapeutic opportunities for patients are exciting, the stakes for patients and drug developers are high. We want to be your partner, add…
Rentschler Biopharma: Excellence Driving Us Forward
Rentschler Biopharma SE, a leading contract development and manufacturing organization (CDMO), has been at the forefront of biologic development and manufacturing for over 40 years. Our value chain encompasses the whole process: from gene to vial and from concept to market. As a full-service provider, Rentschler Biopharma offers its clients one primary contact, with only one contract signed for all phases of a development cycle. As a provider of high-end solutions for our partners, we help clients transition a project…
The Microbial CDMO: Process Development and Manufacturing of Biologics
Wacker Biotech is “The Microbial CMO” — the partner of choice for process development and contract manufacturing of biopharmaceuticals (proteins, vaccines, and live microbial products) using microbial hosts. WACKER’s integrated service portfolio covers molecular biology, process/analytical development, and good manufacturing practice (GMP) manufacturing of biologics for clinical and commercial supply. WACKER operates three state-of-the-art GMP facilities located in Europe (Germany and The Netherlands). Manufacturing lines with two 300-L and two 1,500-L stainless steel fermentation vessels, single-use fermentation lines, matching downstream…
Rapid Help for Cancer Patients: Fully Integrated Project Solution for Accelerated Supply of Valuable Active Agents to the Market
The development of a new, innovative drug is a highly complex and cost-intensive technical task. It normally takes more than 10 years from initial idea to first approval of a drug. As the statutory requirements for patient safety have become ever more stringent in recent years, development expenditure has increased significantly. High investment costs needed to develop some drug products are estimated to be 1–1.6 billion US dollars, which can be amortized only after market launch (1). The race against…
Process Effects on Drug Product Quality in Pharmaceutical Manufacturing: A Validated Measurement Process
Pharmaceutical manufacturing is an industry laden with many challenges and risks because of the life-changing nature of its products — drugs that can affect millions of human lives. Manufacturers perform immensely complex research and testing daily while facing economic challenges and regulatory requirements. This article covers the benefits of surface plasmon resonance (SPR) in the drug development pipeline and describes a recent validation guide specifically for the implementation of SPR technology in regulated workflows. Fill out the form below to…
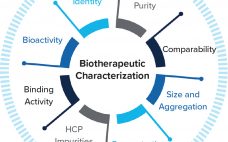
Antibody Affinity Extraction Enables Identification of Host Cell Proteins by Mass Spectrometry
Host cell proteins (HCP) constitute a major group of impurities for biologic drugs produced using cell culture technology. Even at nanogram per milligram concentrations of HCP to drug substance (DS), HCPs can elicit undesired immune response, interfere with drug safety and efficacy, or impact DS stability. A broadly-reactive HCP ELISA should be used during the purification processes to ensure removal of HCPs and to demonstrate process consistency and final DS purity. Regulatory authorities are requesting biopharmaceutical companies employ orthogonal methods…