The manufacture of vaccines and therapeutic proteins has suffered from a reputation of being part art and part science, with heavy doses of regulatory uncertainty thrown in. Postapproval changes (PACs) to chemistry, manufacturing, and controls (CMC) were initiated reluctantly and carefully in the era of “the process is the product.” Today, CMC PACs are a normal part of the biopharmaceutical industry business. Emma Ramnarine (head of global biologics quality control at Hoffmann-La Roche in South San Francisco, CA) notes that…
2018
Addressing the Risk of Bioburden and the Need for Increased Productivity in Protein A Chromatography
Protein A has been a fantastic tool for the antibody industry. It is without doubt the most well-established purification technique in monoclonal antibody (MAb) manufacturing today due to a few key success factors. Protein A is the result of the long evolution of Staphylococcus aureus that developed a defense system against antibodies. Protein A exists on the cell wall of about 9% of S. aureus strains and immobilizes IgGs. When there is an immune response in the body, the bacterium…
Evaluation of a Next-Generation Protein A Chromatography Resin for the Purification of Monoclonal Antibodies
Next-generation, high-capacity, alkali-stable protein A resins have recently become available in anticipation of increased production and throughput demand for protein therapeutic processes. Efficient caustic cleaning and bioburden control regimens have allowed agarose-based, alkali-stable protein A chromatography resins to become the backbone of many commercial purification processes for over a decade and have served as the primary capture step for monoclonal antibody (MAb) purification processes. However, the relatively high raw material costs, along with limitations to binding capacity and aggressive resin…
Reimagining Capacity for Today’s Purification of Monoclonal Antibodies
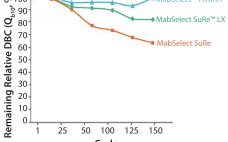
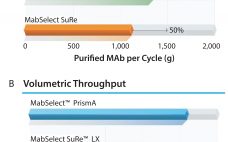
The monoclonal antibody (MAb) market has grown over the past decade to be about half of the biomanufacturing market today. This growth should continue, driven by a strong pipeline of MAbs that are currently in phase 2 and 3 clinical trials. A synergistic evolution of MAbs and protein A resins also has taken place in the market. Figure 1 shows a graph adapted from an Amgen study of the development of protein A productivity and capacity over the past 40…
Thirty Years of Monoclonal Antibodies and Protein A: A Retrospective
In 1980 at the University of Cambridge’s department of pathology, I worked with Herman Waldmann to develop monoclonal antibodies (MAbs) as treatments for graft-versus-host disease (GVHD). That disease is associated with serious complications of stem cell transplantation when attacking T-cells can damage the lungs, liver, skin, and other organs. If we could find a specific MAb that would work with the human complement system to kill those cells, they could be selectively removed from the bone marrow. The human complement…
A Discussion Regarding the Productivity in Downstream Operations
At the Biotech Week Boston conference (24–28 September 2017), BioProcess International publisher Brian Caine had the opportunity to speak with Jonathan Royce, global business leader for chromatography resins at GE Healthcare. Their discussion covered current downstream challenges and the company’s next-generation chromatography resin. Industry Challenges Caine: What are the most critical purification challenges for biomanufacturers? Royce: The bioindustry has seen more than a 100-fold increase in the productivity of cells. This means that downstream purification technology must continue to evolve…
eBook: Challenges Facing Biosimilar Entries into US Markets
Since the 2009 enactment of the Biologics Price Competition and Innovation Act (BPCIA) (1), the US Food and Drug Administration (FDA) has licensed six biosimilar products under PHS 351(k) and approved one product under FD&C 505 (b)(2). It also provided complete response letters (CRLs) to four biologics license application (BLA) filings (Table 1) (2). By comparison, the European Medicines Agency (EMA) has approved 31 biosimilar products (3) and refused or withdrawn about five. There is no doubt that US market…
High-Throughput Technologies: Accelerating Process Development
One of the key elements of any biopharmaceutical drug development project is the timeline from identification of the appropriate DNA sequence to investigational new drug (IND) application filing and the start of clinical trials. Typically, this timeline ranges from 18 to 20 months, depending on the type of molecule being developed and the extent and requirements of the chemistry, manufacturing, and controls (CMC) packages supporting the nonclinical and clinical parts of a development program. There is constant pressure to shorten…