Launched in June 2018, the BioProcess Insider digital information portal delivers the latest financial and business news and expert insider views influencing the commercialization of biopharmaceuticals. Here are just a few recent stories edited for our space limitations in print. For more discussion and in-depth analysis, check out the website at www.bioprocessinsider.com. Every edition provides expert and insider perspectives on current financial movements and deal-making; the newest technology purchases and capacity investments; regulations affecting the bioprocessing sector; global market actions…
2018
A Multistep Research Protocol to Develop and Implement Validated Guidelines for CMO RFI and RFP Processes: Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)
Pursuant to the proposal for validated minimum standards for biopharmaceutical contract manufacturing organization (CMO) request-for-information (RFI) and request-for-proposal (RFP) processes (biopharmaceutical vendor evaluation and selection minimum standards, BioVesel) (1), we propose herein a multistep research protocol to develop and implement the BioVesel standards. This proposal is intended as a basis for discussion among mulitple stakeholders. Detailed research protocols for each proposed stage in the development and implementation of BioVesel will be drafted and published separately. The context of the proposed…
Building a Bridge Across the ‚ÄúValley of Death‚ÄĚ: Strategies to Help Support Technology Development
On Thursday 6 September 2018 at the annual BioProcess International Conference in Boston, the first ‚ÄúTechnology Round Robin Featuring Six Innovative Bioprocess Technologies‚ÄĚ was presented in an interactive session with attendees as active participants, asking questions and engaging in conversation with the six featured entrepreneurs. Detailed below, this session was a culmination of several steps in an overall strategy for some of the companies participating. To fully appreciate the launch of new technologies into the bioprocess arena, you first must…
Rolling with the ‚ÄėTides: Elucidating the Role of Peptides and Oligonucleotides in the Biopharmaceutical Industry
In earlier issues of BPI we published a few ‚ÄúElucidation‚ÄĚ closers that we called ‚ÄúDefining Moments.‚ÄĚ Since then, we have tried to distinguish key confusable terms from one another. Those presented (and sometimes ‚Äúelucidated‚ÄĚ) have been analytical and bioanalytical, spectroscopy and spectrometry, and biosimilars and biobetters. They are just a few of the many confusable terms in the biopharmaceutical industry. For example, when someone says ‚Äúdrug delivery,‚ÄĚ a formulator will think of a syringe or transdermal patch, but a logistics…
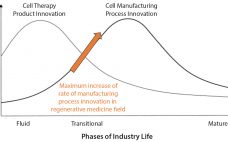
The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics
Most commercial biopharmaceuticals originated from academic research laboratories and start-up development laboratories. Despite such products having differences in modalities and targeted disease indications, and whether their target patient populations are relatively small or approaching blockbuster status, at a key point in development, biopharmaceutical production must scale up from laboratory to commercial production. That movement from research to development and then to manufacturing forces attention on economics and speed to market, and it drives innovative approaches to producing biopharmaceutical cell compositions…
Development and Application of a Simple and One-Point Multiparameter Technique: Monitoring Commercial-Scale Chromatography Process Performance
In commercial-scale biopharmaceutical manufacturing, downstream chromatography steps are still a bottleneck and contribute to significant operational costs (1, 2). Some of those costs are inherent (e.g., resins, large buffer quantities, and cleaning) whereas others are avoidable (e.g., product loss due to rejected lots or deviations that result in production downtime). Maintaining efficient and robust chromatography process performance is therefore critical for minimizing operating costs. To do so, we introduce a simple and one-point multiparameter technique (SOP-MPT) for monitoring chromatographic process…
Speeding Characterization of Biologics: Replace Traditional Assay Technologies with Label-Free Quantification and Kinetics
Fort√©Bio‚Äôs Octet instruments are an ideal replacement for ELISA, HPLC, and SPR techniques in quantification of antibodies and recombinant proteins and in testing product potency for lot release. Bio-Layer Interferometry (BLI) technology monitors biomolecular interactions in real time to determine affinity, kinetics, and concentration. The plate-based, microfluidics-free format offers users several distinct advantages over other technologies. BLI-based systems can achieve higher throughput, with the flexibility to measure two to 96 samples simultaneously. Lower maintenance requirements and increased ease-of-use further shorten…
Elucidation: Changes in VA Healthcare Pose New Implications for Drug Companies
Veterans of the US military still struggle to access healthcare despite the 2014 congressional passing of the Veteran‚Äôs Choice Program (VCP), a US$10 billion-dollar ‚Äúfix‚ÄĚ that allows qualifying veterans to see community physicians who have contracted with the Department of Veterans Affairs (VA) to provide care. Veterans who enrolled in VCP to avoid long wait times at department medical facilities still have faced month-long delays before seeing a doctor, according to a 2018 GAO report. Investigators have found that the…
Ask the Expert November: Expediting Characterization and Maximizing Reliability of Process Validation
On 24 October 2018, BPI presented a free ‚ÄúAsk the Expert‚ÄĚ webinar with Abel Hastings, director of process sciences at Fujifilm Diosynth Biotechologies. He discussed the use of systematic tools to expedite process characterization and maximize reliability of process validation campaigns. Hastings’s Presentation As a project moves from clinical manufacturing toward process validation ‚ÄĒ and ultimately toward preapproval inspections ‚ÄĒ project timelines can become hypervisible at all levels of an organization. Missteps can be costly. The commercial viability of a…
Ask the Expert November: Improving CHO-Cell Biomanufacturing with a Whole-Genome CRISPR Screening
On 18 October 2018, BPI presented a free ‚ÄúAsk the Expert‚ÄĚ webinar with Jamie Freeman, a bioproduction product manager at Horizon Discovery. His company is developing a whole-genome screen using clustered regularly interspaced short palindromic repeats (CRISPR) for improving the capacity of Chinese hamster ovary (CHO) cells in biomanufacturing. Freeman‚Äôs Presentation Freeman described the screening approach that Horizon developed to improve its own CHO cells. It also may be used to improve other such cell lines for biomanufacturing. Founded 11…