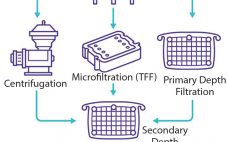
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
2018
Ensuring the Integrity of Single-Use Containers: Providing Robustness, Science, and Helium-Based Technology with a Detection Limit of 2 ÎĽm
Identifying the greatest defect size, both for liquid leaks and microbial ingress, is a fundamental step toward protecting the integrity of single-use systems (SUS) under real process conditions. Integrity testing of such systems may become a prerequisite in the future because they are used in the most critical process steps, with detection limits correlating to liquid leaks and microbial ingress. Such testing guarantees the sterility of drug substances and drug products packaged in single-use systems and, therefore, enhance patient safety.…
Your Brand Is the Patient’s Experience
The future success of biopharmaceutical businesses will depend at least partly on their ability to create meaningful brand experiences from the start of a drug program. By “brand,” I don’t mean logos and taglines. I’m talking about meaningfully unique experiences that directly affect clinical and patient needs — specifically, to address the growing demand for self-administered injectable therapeutics. Whether you are a biosimilar developer trying to carve out differentiated value or a market leader looking at your patent protection in…
Ask the Expert: Integration of Cell Line, Process, and Analytical Technologies: Speeding Development and Clinical Supply of Emerging Therapy Products
Chinese hamster ovary (CHO) cells are being pushed to their productivity limits in drug development and biomanufacturing. Biopharmaceutical companies working on promising new therapies increasingly struggle with development challenges such as weak protein expression, low-yield purification steps, and poorly optimized analytical techniques. To address those challenges, innovators need robust cell-expression platforms and advanced process, analytical, and biomanufacturing technologies. Since 2012, KBI Biopharma has performed development and/or manufacturing services using more than 20 different cell lines generated by Selexis SA. In…
eBook: Innovations in CRISPR Technology — A Perspective on Research and Bioprocess Applications
One of the fastest growing areas in genome engineering is research using the powerful editing tool of clustered regularly interspaced short palindromic repeats (CRISPR). When paired with the Cas9 (CRISPR-associated protein 9), an RNA-guided DNA endonuclease enzyme from Streptococcus pyogenes, the site-specific prokaryotic immune system can be used to cut and manipulate DNA strands in cells of patients with genetic diseases to treat, or in some cases, prevent such diseases. Within the past couple of years, CRISPR has been shown…
Cost Analysis of Cell Therapy Manufacture: Autologous Cell Therapies, Part 1
Cell therapies are a growing area of interest for the treatment of a number of indications such as neurological, cardiovascular, and ophthalmological maladies that are refractory to other more conventional drug therapies. A number of cell-based therapy products currently are undergoing clinical trials. The most common target is oncology, which represents 46% of all cell-based therapies through the use of traditional blood-cell and immune-cell–based therapies. For treatment of various cancers, immune cells, lymphocytes (natural killer cells, T cells, and B…
Sourcing Clinical-Grade Human Tissue: Considerations for Supporting Cell Therapy Development and Production
The rapidly developing global cell therapy market poses numerous industry challenges for drug development, process scalability, commercialization, and patient safety. The processes of procuring donated human tissue for clinical applications are fraught with many technical, ethical, and legal issues. Allogeneic cell therapies involving primary cell types such as bone marrow mesenchymal stromal/stem cells (BM-MSCs), hematopoietic stem and progenitor cells (HSPCs), and T and natural killer (NK) cells for immunotherapy applications are especially challenging because of the vigorous process of screening…
Driving Cell Therapy Innovation: Applying Key Lessons from the Evolution and Commercialization of Protein-Based Therapies
After many trials and errors — and milestones — regenerative medicine has become a mainstream part of the biopharmaceutical industry, supported by at least 670 companies and clinics of all sizes. But many experiences in the protein-based industry segment can be leveraged to further improve successful commercialization of advanced therapies. At the 2017 Biotech Week Boston conference, BioProcess International editor in chief Anne Montgomery hosted a panel of industry cell therapy experts to discuss key lessons that can be gleaned…
Cell Expansion with Dissolvable Microcarriers
In recent years we have seen an exponential increase in the number of companies testing and validating new regenerative medicine products. Many of these products are reaching late-phase trials with the potential to receive final approval and marketing authorization from regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). In the past several years, we have seen successful launches of regenerative medicine products, including Holoclar (Holostem Advanced Therapies), Kymriah (tisagenlecleucel, Novartis), Yescarta…
March From the Editor
Are you protecting your trademarks? Are you sure? You’ll notice that BPI doesn’t use registration marks (e.g., ™ and ®) anywhere but in advertisements and the occasional “advertorial” piece. Ever wonder why? According to the official stylebook of the American Chemical Society, which is the basis of our “house style,” they are entirely unnecessary. And editors everywhere dislike the impression they give as well. On page 157 of the ACS stylebook, trademarks (“brand names”) are defined as adjectives that describe…