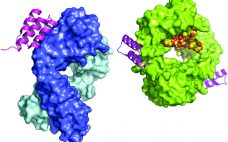
Protein A affinity chromatography is a well-established technology that is used extensively for large-scale purification of monoclonal antibodies (MAbs). With this mode of chromatography, very high product purity can be achieved in a single, relatively simple unit operation. A solution containing the target protein of interest is applied to a liquid-chromatography column at near-neutral pH, and one or more wash steps follow to lower product- and process-related impurities (1). Product is eluted through application of a low-pH buffer. Finally, the…
2018
Statistical Properties of WECO Rule Combinations Through Simulations
The practice of quality control is promoted by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). A number of process control charts are used in many biopharmaceutical companies to increase production and lower process costs. Here we focus on performance of three commonly used control charts: Shewhart control charts with WECO and supplementary rules, monitoring charts with exponentially weighted moving average (EWMA), and cumulative sum (CUSUM) charts. WECO rules got the name from a quality control…
Innovators and Biosimilar Companies: Experts Predict Intense Conflicts Ahead
CPhI Worldwide (organized by UBM) announced last fall the final section findings of the fifth edition of the CPhI Annual Report. Presented live at the meeting in Frankfurt, Germany, the report is now available online. It highlights immediate and long-term trends in pharmaceutical data, regulation, generics, and biosimilars. Four experts gave their views. They warned that the US Food and Drug Administration’s (FDA) approach to achieving six-sigma (nearly perfect or 99.9997% defect-free) quality is failing in respect to the pharmaceutical…
eBook: Raw Materials Quality, Processing, and Storage — A Manufacturing Case Study
Raw material storage, handling, and processing are essential to ensure high product quality and consistent process performance. Slight variabilities in raw materials (either inherent in the material or through processing) can compromise yield and even result in batch loss. On Tuesday 26 September 2017 speakers at the BioProcess International Conference (part of Biotech Week Boston) addressed raw material variability and control strategies in biomanufacturing. They discussed the industry’s initiative for raw material risk assessments and strategies to control variability by…
eBook: SUStainability — Concerning Single-Use Systems and the Environment
Disposable materials have been used in many aspects of biomanufacturing since muromonab was first launched in 1986. Single-use stirred-tank bioreactors first became commercially available from HyClone in 2004 (1). Despite their demonstrated value to bioprocessing, disposable materials remain the subject of wide-ranging differences of opinion. Discussions of any technology are healthy and important for identifying areas for improvement, but some hearsay and bold propositions made regarding single-use components and the environment are not always helpful. Sustainability is an important and…
May 2018 From the Editor
So many manuscripts we receive begin by mentioning the “ever-growing” or “ever-expanding world of biopharmaceuticals.” For anyone who’s been covering this industry for a while, that’s an understandable impression — but it’s not always easy to express. Despite increasing in maturity, that “world” remains on the cutting edge of science and technologies for innovative therapeutic development, and its entrepreneurial spirit remains strong. But we’re all accessing information in different ways now than in the past. We need and want that…
May Spotlight
Welcome New BPI Colleague In April, BioProcess International waved a reluctant goodbye to European strategic marketing consultant Joanna Hendrikx. Pregnant with her second child, she is looking forward to devoting herself exclusively to motherhood. We will miss her energy, finesse, and sense of humor even as we welcome our new colleague taking over her role here. Victoria Biscoe has a long history with Informa, our parent company, having joined in 2010 as a key account manager for the Informa Life…
A Product–Packaging Interaction Study to Support Drug Product Development
Drug packaging is subject to a number of regulatory requirements, including those for product containers and packaging. For example, according to the federal Food, Drug, and Cosmetic Act (FD&C) section 501(a)(3), a drug is considered adulterated “if its container is composed, in whole or in part, of any poisonous or deleterious substance which may render the contents injurious to health.” And 21 CFR states that drug packaging “shall not be reactive, additive, or absorptive so as to alter the safety,…
Host-Cell Protein Risk Management and Control During Bioprocess Development: A Consolidated Biotech Industry Review, Part 1
Host-cell proteins (HCPs) constitute a significant class of process-related impurities during biologics manufacturing. Due to their potential impact on product quality and efficacy as well as patient safety, the total amount of residual HCP in a biological drug substance generally is considered a critical quality attribute (CQA) that usually needs to be tested for during batch release (1, 2). It is both an “industrywide” common understanding and a regulatory requirement to remove HCPs from biologics to acceptably low levels that…
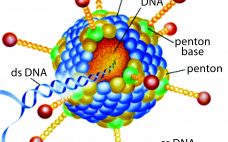
Development of a Freeze-Dried Ebola-Expressing Adenoviral Vector: Unexpected Findings and Problems Solved
In December 2013, a two-year-old child in Guinea became the first person to be killed by Ebola in the most recent outbreak. In March of the following year, that outbreak was declared in West Africa. By mid-2014, the World Health Organization (WHO) had declared it to be a public health emergency of international concern and urged pharmaceutical companies to accelerate their development of candidate vaccines. At the peak of the outbreak in 2014, more than 1,200 new cases of Ebola…