As the need to accelerate biopharmaceutical development around the world continues to grow, biomanufacturers face a host of challenges so they, too, can grow. Increasing process productivity, reducing cost, mitigating risk, and bringing products to market faster are just a few of the issues frequently addressed. But with support in process development, cGMP manufacturing and training, accelerating bioprocess development can become less challenging. Several biomanufacturers have successfully navigated these issues in collaboration with GE Healthcare’s Fast Trak Services. Whether…
2017
Introduction: Fast Trak Services Accelerate Global Biopharmaceutical Development
As the need to accelerate biopharmaceutical development around the world continues to grow, biomanufacturers face a host of challenges so they, too, can grow. Increasing process productivity, reducing cost, mitigating risk, and bringing products to market faster are just a few of the issues frequently addressed. But with support in process development, cGMP manufacturing and training, accelerating bioprocess development can become less challenging. Several biomanufacturers have successfully navigated these issues in collaboration with GE Healthcare’s Fast Trak Services. Whether it…
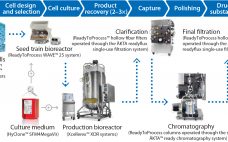
Flavivirus Vaccine Production Accelerates with Modern Bioprocess Tools and Solutions
As with all viral vaccines, the complex nature of flaviviruses makes process development technically challenging. In addition, vaccine production can be both costly and difficult to scale to meet market demands. In egg-based vaccine production, for example, 100–300 vaccine doses can be produced from one fertilized hen’s egg. However, the eggs used for production need to be supplied from special pathogen-free chicken flocks, limiting availability of eggs and making vaccine production difficult to scale up. To meet the needs of…
Pfizer Collaborates to Accelerate Approval of Biosimilar MAbs in China
In their pursuit of developing therapeutics, biopharmaceutical companies face many challenges, including pressure to meet aggressive timelines, reduce risk and cost, and increase speed to market. Addressing these challenges is especially critical for biosimilar manufacturers, who face fierce competition and an expanding footprint in emerging markets (1). The global biosimilars market is growing rapidly and is expected to exceed more than US$6 billion by 2020 (2). This case study describes how Pfizer is collaborating with GE Healthcare to achieve regulatory…
Roivant Sciences Accelerates Process Development to Speed Orphan Therapy to Market
Although the demographic of orphan therapies is small, making therapies for rare diseases available has a huge impact for the affected patients. Cooperation to expand capacity and expertise during process development and manufacturing for preclinical and clinical phase studies is one way to increase speed to market. This case study shares the work of GE’s Fast Trak Services team to help accelerate development of a process for cGMP production of material for toxicology studies. Frequent communication between the Fast Trak…
MAbxience Optimizes and Accelerates Downstream Biosimilar Process Development
Biosimilars represent an innovative solution that can benefit both patients and healthcare systems by reducing the burden of rising treatment costs. To improve the availability, price, and access of medicines, many countries are implementing strategies to establish their own production capacity. To support such development, MAbxience (a Spanish biotechnology company specialized in research, development, and manufacture of biosimilar drugs) is committed to provide the manufacturing of high-quality products and processes that meet regulatory and technical requirements in all countries where…
Advanced Control Strategies at Biotech Week Boston
Attendees at this year’s Biotech Week Boston (24–28 September) had the opportunity to participate in several preconference symposia on the first day, including one on advanced control strategies for bioprocessing and biomanufacturing. Chaired by William Whitford (GE Healthcare), the session included presentations from Dan Kopec (Sartorius Stedim Data Analytics), Markus Gershater (Synthace), Jonathan Bones (National Institute for Bioprocessing), Robert Thomas (Loughborough University), Chris McCready (Sartorius Stedim Data Analytics), and Victor Konakovsky (Newcastle University). BPI has collaborated with conference organizer KNect365…
Integrated PAT Automated Feedback Control of Critical Process Parameters Using Modern In Situ Analytics
Simply put, the best way to control a critical process parameter (CPP) is to measure that specific parameter, integrate the live signal into your control system, and apply a smart feedback algorithm for an automated control loop. The challenge in doing this for bioprocesses has been due, in part, to the complex, highly dynamic, and variable nature of the process along with the lack of robust, scalable, and multiformat (single-use or multiuse) technologies that can monitor (in real time) such…
Accelerating Process Development Through Flexible Automated Workflows
Synthace began as a bioprocess optimization company in 2011, spun out of University College, London. The company worked on multifactorial approaches with 15–30 factors simultaneously instead of seven or eight. The work investigated genetic strain engineering factors alongside process parameters, defining deep interactions between the way strains were designed and the way they were treated in bioprocesses. Those complex experiments gave unique insight into the complexities of biological processes, but they were exceptionally taxing to plan and carryout manually. Automation…
Model Predictive Control for Bioprocess Forecasting and Optimization
Automation hierarchy in bioprocess manufacturing consists of a regulatory layer, process analytics technology (PAT), and (potentially) a top-level model-predictive or supervisory layer. The regulatory layer is responsible for keeping typical process measurements such as temperature, pressure, flows, and pH on target. In some cases, spectral instrumentation in combination with multivariate analysis (MVA) can be configured to measure parameters such as glucose concentration. A cascade control structure can be set up when the nutrient flow setpoint is adjusted to maintain the…