“Today we recognize that, to successfully protect US public health, we must think, act, and engage globally. Our interests must be broader than simply those within our own borders.” —Margaret Hamburg, US Food and Drug Administration (FDA) commissioner, 2009–2015 Many major pharmaceutical companies — including Pfizer, Novartis, Eli Lilly, Sanofi, and Bayer — have research and development centers as well as clinical and commercial activities in China. Their sales revenues are increasing with the rapid growth of China’s healthcare market,…
2017
Ask the Expert: Evaluation of the “Scale-Out” Biomanufacturing Strategy — from Early Clinical Stage to Commercialization
In a BPI Ask the Expert webinar on 2 November 2017, Jie Chen (vice president of CMC management at WuXi Biologics) spoke about scale-out biomanufacturing strategies. Chen’s Presentation Scale-out strategies allow for the use of disposable bioreactors in commercial manufacturing. A scale-out strategy offers unique advantages over traditional scale-up manufacturing: Reduces risks to product quality and process performance as manufacturing scale increases Allows for flexible process design and validation strategies Accommodates a wide range of productivity levels and market demands…
Control of Single-Use System Supply Chains
Single-use technologies are now dominant for the clinical production of biopharmaceuticals and are becoming more mainstream within commercial manufacturing facilities. They allow biologics manufacturers to decrease the footprint of their facilities by approximately 20% because of a reduced need for utilities that generate water, steam, and clean-in-place solutions. Engineers believe that the capital outlay for a single-use facility is 25–45% less than for a facility based on stainless steel equipment. Similarly, they estimate that such facilities need half the water…
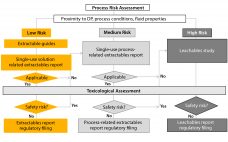
Exploring the Science Behind Single-Use Container–Closure Integrity Assurance
Failures in the integrity of single-use systems during commercial manufacturing can cause a number of serious problems for biomanufacturers. A loss of system integrity during processing can allow environmental contaminants that can be dangerous for patients (e.g., microbes) to enter a process. Biopharmaceuticals and their intermediates can be highly potent or even infectious agents, so an integrity failure can jeopardize the safety of operators. In severe cases when biomanufacturers cannot ensure the quality of drug products for fear of a…
Single-Use Production Platforms for Biomanufacturing
The pipeline of biopharmaceuticals remains strong, and the market for biologics could exceed US$450 billion by 2025. Analysts predict that sales within segments such as regenerative medicine and antibody–drug conjugates (ADCs) will grow faster than 20% each year. Yet considerable challenges remain for biopharmaceutical companies to overcome if they wish to be successful. First, they must reach the market quickly. Analysis by the Boston Consulting Group shows that the proportion of available value that a newly launched product can capture…
Control of Critical Process Parameters Using In-Situ Analytics
Biopharmaceutical companies can develop entire end-to-end single-use production platforms and use them for commercial manufacture of their biological products. Single-use facilities are flexible, can be implemented quickly, and do not require the large up-front capital investments needed for stainless steel equivalents. However, single-use facilities must be supplied with a large quantity of high-quality consumables. Biomanufacturers should pay close attention to their supply chains for those consumables to ensure that they are robust, integral, and fully compatible with biological expression systems…
Prescriptive Analytics for Bioprocessing Platforms
The preceding articles have shown how biopharmaceutical companies increasingly are adopting single-use manufacturing technologies for commercial production facilities as their confidence grows in the material science, supply chains, and robustness of single-use systems. Single-use suppliers can support adoption of end-to-end process platforms in commercial manufacturing settings with dedicated teams of experts in process development, engineering, and regulatory support. Advances in process analytical technology (PAT) are providing engineers with greater information on conditions within their single-use bioprocess platforms to allow for…
Final Thoughts: Single-Use Platforms
The development and launch of new biopharmaceutical products is a very challenging and risky process. Sponsors must get their products into clinical testing as quickly as possible to beat their competition and take the greatest share of the market. Yet that need for speed must not lead to companies launching products with inefficient manufacturing processes that ultimately will leave them vulnerable to attack from low-cost competition. Single-use systems have been a significant enabling technology for companies developing new biologics because…
eBook: Advances in Single-Use Platforms for Commercial Manufacturing
This special report from Sartorius Stedim Biotech explores recent advances in single-use process platforms for commercial production of biopharmaceuticals. Enormous developments have been made in single-use systems, allowing biomanufacturers to adopt the technology in critical applications within their commercial-scale facilities. The authors illustrate how companies can work with teams of experts from supplier organizations to implement end-to-end single-use bioprocesses at scales up to 2,000 L by carefully considering the approach from the earliest stages in process development. Finally, the report…
Are All Protein A Resins the Same? A Performance Comparison of Eight Different Protein A Resins
Protein A affinity chromatography continues to be the preferred method for commercial purification of antibodies due to its very high selectivity and robust resin performance over repeated purification cycles. It is now estimated that over US$125 billion of yearly sales will be generated from monoclonal antibody (MAb) products by 2020 (1). The clear majority will be purified by large-scale protein A affinity chromatography. With the continued growth and therapeutic commercial importance of MAb production, the availability of high-quality resin material…