The US Food and Drug Administration has stated its appreciation of continuous bioprocessing (CBP), and some studies have shown that it can save manufacturers time and money. However, the bioprocessing industry is still reluctant to implement continuous bioprocessing right away. It will be interesting to see which companies will be among the first-movers to harness the competitive benefits. Although few biologics today are made using CBP-enabled equipment (e.g., advanced bioreactors), the industry is changing. For biologics already in production, it…
2017
BioPhorum Operations Group Technology Roadmapping, Part 3: Enabling Technologies and Capabilities
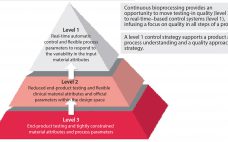
Although great strides have been made over the past 20 years to increase the productivity and robustness of manufacturing processes for biopharmaceuticals, the cost and complexity of their development and manufacturing remain high, especially in comparison with those of small-molecule pharmaceuticals. Process improvements are required to increase patient access while maintaining the viability of an R&D-driven biopharmaceutical industry. Facility productivity, cost of goods (CoG), and capital investment all have significant margins for improvement. Such goals can be achieved not only…
Aggregation from Shear Stress and Surface Interaction: Molecule-Specific or Universal Phenomenon?
Exposure to solid–liquid and air–water interfaces during production, freezing and thawing, shipment and storage of protein therapeutics may be a contributing factor in their degradation (e.g., aggregation, fragmentation) (1, 2). Surface exposure, particularly during manufacturing processes, often is accompanied by various degrees and durations of shear stresses originating from fluid flow and acting on proteins at interfaces. The magnitude and duration of shear rates depends on velocity gradients within each solution and varies significantly among manufacturing steps. On the low…
Strategies for Successful Sample Transfer
Nadine Ritter is president and senior analytical advisor of Global Biotech Experts, LLC and a long-time member of BioProcess International’s editorial advisory board. At a recent CASSS North American CMC Strategy Forum called “Methods on the Move: Addressing Method Transfer Challenges,” she discussed the biopharmaceutical industry’s logistical challenges of analytical test samples for drug substances and products. At the conference, BPI’s editor in chief Anne Montgomery met with her to discuss some key points of this topic. Logistics Challenges Montgomery:…
How to Set Up a Perfusion Process for Higher Productivity and Quality
Biotherapeutic proteins usually are produced by either fed-batch or perfusion processes. Perfusion manufacturing can provide much higher levels of productivity than fed-batch systems can, thereby reducing production costs. A 2013 study showed that perfusion is more cost effective than fed-batch processes for most combinations of titers and production volumes (1). Moreover, because a perfusion process is much closer to steady state than is a fed-batch process, it often produces a more consistent product — especially for molecules that are sensitive…
Enabling Faster Workflows with Protein Purification Technologies: Improvements in Chromatography and Electrophoresis
Purification of recombinant proteins is a critical step during protein therapeutics development. Protein therapeutics have a number of classifications based on their potential applications, including use as vaccines and diagnostics as well as for enzymatic, regulatory, or targeting activities (1). For all such applications, identifying and verifying protein purity is most important. Whether proteins themselves are therapeutics or the target proteins of interest, effective purification is essential in drug development. Since the introduction of recombinant proteins in the early 1980s,…
Going After a Moving Target: New Production Methods Aid in the Flu Fight
The traditional method of manufacturing vaccines for influenza involves infecting hens’ eggs with the virus, then harvesting and purifying the large amounts of virus that they produce as a result. It’s time-consuming and expensive, requiring large specialized facilities for production. With the advent of genetic engineering and decades of improvement in protein production through cell-line engineering and industrial culture, it was only a matter of time before the vaccine industry saw the real value in modern biomanufacturing instead (1, 2).…
Biosimilar Markets and Regulation: Which Countries Are Going All In?
The pipeline of follow-on (biosimilar and biobetter) products in development for the US, EU, and other major markets is very healthy. It includes nearly 800 biosimilars, about three-quarters of which are presumed to be targeted for major markets, and about 500 biobetters in development. Nearly 1,200 follow-on biopharmaceutical products in the development pipeline are intended to compete with more than 100 currently marketed biopharmaceuticals. This is not just an opportunity in the Western world; biosimilars development is expanding globally. But…
The Clinical Side of Biosimilar Development
Biosimilars have become common on pharmacy shelves in Europe. The first biosimilar product — Sandoz’s Omnitrope version of Lilly’s Humatrope (somatropin) — was approved by the European Medicines Agency (EMA) in 2006. In the decade that followed, more than 20 biosimilars have gained regulatory approval in Europe. The first biosimilar monoclonal antibodies (MAbs) — comparators to Janssen’s Remicade (infliximab) — were approved in 2013. The pace of approvals in the United States has been much slower. The US Food and…
Evolving Bioassay Strategies for Therapeutic Antibodies: Essential Information for Proving Biosimilarity
The modern age of biologics began 35 years ago with the approval of Lilly’s Humulin product — a biosynthetic form of human insulin derived from recombinant DNA and microbial cell culture (1). Today, about a quarter (27%) of the drugs approved yearly by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) are biopharmaceuticals: primarily monoclonal antibodies (MAbs), but also vaccines, blood products, and (recently), advanced therapies based on genes and cells. A decade ago, the average…