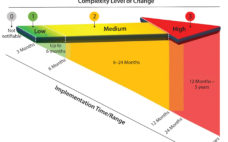
Current practices for change notification in the biopharmaceutical industry are neither efficient nor conducive to accelerating the adoption of single-use systems (1, 2). Drug manufacturers (end users) often observe that supplier change data packages lack technical content or detail and that the time allowed for change implementation is too short. Occasionally, customers (end users or next tier in supply chain) learn of changes after the fact, possibly even by happenstance. Suppliers, however, can find the potential affect of a change…
2017
Opportunities and Challenges in Biosimilar Development
A biosimilar biotherapeutic product is similar (but not identical) in terms of quality, safety, and efficacy to an already licensed reference product. Unlike generic small molecules, it is difficult to standardize such inherently complex products based on complicated manufacturing processes. Table 1 describes the main differences between biosimilar and generic drug molecules. The global biosimilar market is growing rapidly as patents on blockbuster biologic drugs expire (Table 2) and other healthcare sectors focus on reduction of costs. Biologics are among…
Implementing Quality By Design in Analytical Development: A Case Study on the Development of an Anion-Exchange HPLC Method
The concept of quality by design (QbD) initially was outlined in ICH Q8 guidance for drug-product development and later in Q11 for drug-substance development (1, 2). Since then, the QbD concept was further expanded to the development of analytical methods. FDA issued a 2015 guidance on analytical procedures and method validation for drugs and biologics (3). Although the agency did not explicitly state the requirement for implementation of QbD in analytical method development, the concept is embedded in its section…
Scaling Considerations to Maximize the High-Area Advantage
Maximizing filtration-area density is a design strategy to minimize filter footprint and improve filtration process economics. Pleated membrane formats commonly are used to achieve that goal for sterilizing-grade filters operating in dead-end mode (also known as normal-flow filtration). Although high-density pleat geometries increase productivity for a device, such formats can present unique challenges. One of the most common concerns is that pleat formats can introduce flow resistance that impedes a device’s filtration efficiency, particularly for high–pleat-density geometries (1, 2). Filtration…
Current Thinking in Viral Safety: Risk Management Protects Patients
BPI’s editor in chief S. Anne Montgomery recently caught up with long-time editorial advisor Hazel Aranha (purification technologies technology expert for Sartorius Stedim Biotech, North America). They discussed a number of topics related to viral safety. Montgomery: What is the current thinking regarding virus-safety assurance in biopharmaceutical manufacturing? How is the industry preventing viral contamination? Aranha: The “holy grail” of viral safety — absolute freedom from extraneous agents or residual pathogenicity — is a myth. That said, biopharmaceutical products have…
Ask the Expert: Advanced Materials for Single-Use Biomanufacturing Systems
Advanced fluoropolymer materials offer distinct advantages in single-use systems regarding chemical compatibility, extractable levels, and cold-temperature performance. In a 5 April 2017 webcast, Michael W. Johnson (business development engineering manager for life sciences at Entegris) examined data from pilot-scale testing of a new single-use bag system for freezing, storage, and shipping of formulated bulk biologics. Johnson’s Presentation Factors restricting disposables use in biopharmaceutical development include bag breakage, concerns over extractables and leachables, and material incompatibility. Bioprocessors want new and improved…
Introduction: Tackling the Technical and Regulatory Challenges of Biosimilar Development
In a just a few years, the biopharmaceutical industry has gone from questioning the feasibility of “follow-on biologics” (around the time of BPI’s first issues) to fearing them (when we published our first supplement on the topic in 2013) to the acceptance and strategizing of today. Perhaps because of its more socialized medicine, Europe led the way in biosimilar regulation and approved its first such product nearly 10 years before the first US biosimilar launch in 2015. In between came…
Advancements in Characterizing Therapeutic Protein Higher-Order Structure
Electrospray-ionization mass spectrometry (ESI-MS) is a well-established tool for biotherapeutic analysis. It draws intact proteins or peptide ions into the vacuum of a mass spectrometer, where the ion mass is measured. Electrospray ion-mobility mass spectrometry (ESI-IMS) introduces ions into a low-pressure gas, where the effects of aerodynamic drag reveal their shape. This technique is just emerging as a valuable tool for characterizing intact proteins, even though for a decade it’s been the basis of a commercially available medical diagnostic test…
April From the Editor
The BPI West conference is always a “candy store” of timely presentations and key networking opportunities for an editor who cannot be in all places at once. This year’s event in San Francisco (27 February – 2 March) was no exception. Nearly 900 delegates were joined by 86 exhibitors and 48 poster presentations. I’m inviting a number of speakers to convert their talks into manuscripts for BPI. In focusing on late-stage processes and commercial trends at this year’s event, I…
April Spotlight
WHO’s Most-Wanted List: Deadliest Bacteria In February, the World Health Organization (WHO) published its first-ever list of “priority pathogens,” a catalogue of 12 families of bacteria that pose the greatest threats to human health. In particular this list highlights the threat of gram-negative bacteria that are resistant to multiple antibiotics. It is intended to guide and promote research and development (R&D) of new antibiotics. “This is a new tool to ensure R&D responds to urgent public health needs,” said Marie-Paule…