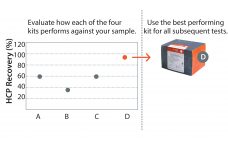
Removal of host cell protein (HCP) impurities in drug substances is critical in the manufacture of highquality drug products and for patient safety. A key requirement is a thorough analysis of HCP contaminants, which may vary considerably depending on cell line, media, and process parameters. How well a particular HCP assay recognizes all HCPs will depend on how well its antibodies match the actual HCP composition, their abundance, and affinities for each HCP. Accordingly, various commercial generic HCP ELISAs may…
2017
Dissolved Oxygen Quantification in a DO-Sensitive Product: Study of DO Values at Laboratory and Industrial Scales
Sulfur is an essential component of all living cells. In plants and animals, the amino acids cysteine and methionine contain most of the sulfur, and the element is present in all polypeptides, proteins, and enzymes that contain these amino acids. Sulfur-containing compounds often show different biological activities and provide important functions in pharmaceutical industry applications. Sulfonamides, thioethers, sulfones, and penicillins are the most common scaffolds in sulfur-containing drugs. Sulfonamide drugs were the first antibiotics to be used systemically, and they…
Nonaffinity-Based IgM Purification Platform
The biological properties of IgM antibodies make them very effective vehicles for in vitro diagnostics and therapeutics. However, purification of IgM antibodies is far more complex than that for IgG antibodies. Furthermore, affinity chromatography is not ideal for purifying IgMs due to the required elution conditions. Here we propose a nonaffinity-based platform for IgM purification. This strategy utilizes a cation exchange (CEX) media, Nuvia™ S, for capture, and a mixed-mode media, CHT™, for polish purification. It is suitable for purification…
Effective Viral Clearance in mAb Purification Using TOYOPEARL® Resins
Impurities such as viruses are typically present during the manufacturing of biological drugs. The viruses may be present in the unprocessed material from the upstream collected harvest, such as released from cell culture media (endogenous) or accidentally introduced during processing (adventitious). Viral clearance is required by the FDA and international regulatory bodies as specified in the ICH Q5A documents. In this study we evaluated two chromatography steps in the purification of a monoclonal antibody (MAb) for viral clearance. Evaluation was…
Identification of Process Parameters Which Underpin Robust Platform Production Processes
Critical objectives for the biopharmaceutical industry are the creation of robust, reproducible processes which result in consistent critical product quality attributes and yields. To meet these requirements within a short time period it is important to apply platform production processes which consist of a common host cell line, expression vector, cell line development process, cell culture media/feed, process control and scale-up methodologies during cell line development, process characterization and cGMP manufacturing. In this study we integrate mathematical based approaches with…
Collaborating to Address the Bioburden Challenge
Bacteria and their byproducts can negatively affect the safety and potency of a biopharmaceutical drug. At a minimum, bioburden contaminations lead to reduced productivity as a result of lost batches and/or deviation investigations. Striving towards a bioburden-free process, biopharmaceutical companies and their suppliers must collaborate. To meet this challenge, GE Healthcare is committed to continuous improvement of its chromatography resins to enable delivery of products without detectable bioburden. For example, development of Protein A resins to withstand high NaOH concentrations…
Purification of Antibody Fragments with Amsphere™ A3 Protein A Resin
The binding mechanism between the engineered C domain of the Amsphere™ A3 protein A (PrA) ligand and a VHH single domain antibody (sdAb) was revealed. Binding sites in the PrA ligand in helices 2 and 3 and in framework regions 1 and 3 of the VHH were confirmed. Identified VHH residues are not involved in antigen recognition. Overlap with a human VH showed the same interaction sites. These results provide insight on why Amsphere A3 is a suitable tool for…
Modular, Single-Use Facilities Increase Biomanufacturing Flexibility
The pipelines of biopharmaceutical companies are becoming increasingly diverse while improvements in cell lines are leading to more productive bioprocesses. These factors are driving new capacity demands for bio-pharmaceutical companies and their CDMO partners. It is becoming increasingly important, therefore, that companies implement innovative, highly efficient, flexible facilities that are capable of meeting these challenging capacity demands. Login to view full PDF version of poster.
From the Editor: BioProcess International Theater
This special insert summarizes our well-attended BioProcess Theater at the BIO International Convention in San Diego this past June. Focusing on emerging therapies and technologies enabled us to touch upon interconnected topics of interest to both BPI readers and the BIO exhibit-hall attendees. Speakers highlighted approaches ranging from analytical development to evolving business and commercial models. On behalf of our publisher, Brian Caine (who creates these programs every year) and our marketing and editorial teams, I thank the speakers and…
Panel Discussion: Emerging Biotherapies and Their Manufacturing Challenges
At noon on Tuesday, 20 June 2017, BioProcess International presented a panel discussion as part of the “Emerging Therapies” session of its BPI Theater at the Biotechnology Innovation Organization’s annual convention in San Diego. Moderated by Patricia Seymour (senior consultant at BioProcess Technology Consultants), this panel comprised Holger Wesche (vice president of research at Harpoon Therapeutics), Richard Snyder (chief scientific officer of Brammer Bio), Marc Better (vice president of product sciences at Kite Pharma), and Paulo Carvalho (associate director of…