Endotoxins are degradation products from dying gram-negative bacteria and complex aggregates of acidic lipopolysaccharides (LPS). Each is composed of lipophilic lipids and hydrophilic polysaccharides. In humans, endotoxins can cause immune responses such as fever (pyrogenic threshold is approximately 0.1 ng/kg body weight). Unlike bacteria themselves, endotoxins are extremely heat and pH stable and therefore withstand sterilization methods. During protein purification, the reduction of endotoxins is one of the most important and difficult steps. It often includes complex purification strategies (e.g.,…
2017
Bioburden: Current Innovations and Practices to Address Microbial Contamination in Downstream Bioprocessing
Bioburden control is an area of serious concern for both manufacturers of biologicals and suppliers to the industry. This white paper considers some of the risks related to downstream processing and presents recent developments by suppliers that help manufacturers mitigate these risks. Topics covered include improvements in raw material quality, equipment design, chromatography resin properties, and ways of working. Challenges specifically related to the sanitization of protein A chromatography resins also are discussed. Bioburden Control in Biopharmaceutical Production The risk…
A Novel Cellufine Mixed-Mode Resin: Cellufine™ MAX IB for Polishing of Monoclonal Antibodies
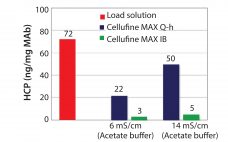
Cellulose is well-known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. Additionally, cellulose particles have unique pore-size characteristics appropriate for the chromatography of biopharmaceuticals. Mixed-mode chromatography resins are well known to have unique selectivity differences from traditional IEX or HIC resins. Cellufine™ MAX IB, a novel cellulose-based mixed-mode resin, has polyallyl amine partially modified with butyl groups ligand (Figure 1). The resin is used in flow-through mode after a protein A step in…
KanCapA™ 3G: An Innovative Protein A Resin for MAb Manufacturing
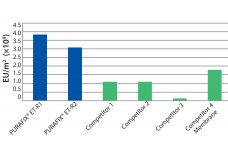
Recent improvements in monoclonal antibody (MAb) upstream process technologies have led to increased product titers (from 5 to 10 g/L) and a corresponding change in impurity levels. To yield highly pure MAb drugs from such high-titer feedstocks, new, robust, and cost-effective purification processes need to be developed to follow this upward trend. KANEKA has developed KANEKA KanCapA™ 3G, an innovative protein A resin that demonstrates higher binding capacities and allows milder elution conditions than well-known high-binding capacity agarose based resin.…
Continuous Chromatography: A Multifunctional, Twin-Column Continuous Chromatography in a Simple, Robust Design
LEWA EcoPrime® Twin LPLC is an easy-to-use, GMP-ready, multicolumn chromatography system offering analytical performance for continuous chromatography from process development to large-scale biopharmaceutical production. The EcoPrime Twin platform spans a wide range of flow and column sizes and has several options to incorporate multiple functionality on a single unit. The EcoPrime Twin system is built on ChromaCon’s Contichrom patented approach to twin-column purification. Because of the two-column configuration, the EcoPrime Twin system is a simple design that facilitates continuous chromatography…
BioSC® in a Fully Integrated Continuous MAb Manufacturing Process
With the demand of higher production and reducing cost in biological manufacturing, continuous bioprocessing is replacing the traditional batch-mode manufacturing. Many biopharmaceutical companies are seeing the benefits of continuous processing. Novasep has enabled fully integrated continuous bioprocessing — from perfusion bioreactor to formulation. Objective This application note highlights a study conducted with Novasep’s BioSC Lab® technology for a fully continuous monoclonal antibody (MAb) production. BioSC Lab® is a sequential multicolumn chromatography (SMCC) system integrated as the capture step using protein…
Reducing Complexity of Custom Single-Use Assemblies
Since the introduction of single-use technologies (SUTs) more than 20 years ago, their benefits have been well recognized, and their adoption has been swift. The ability to customize a solution for every operation, every scale, and every product is an attractive proposition when compared with the fixed, stainless-steel facilities SUTs have replaced. But now customization has gone too far. Custom assemblies are being used when there is no need or benefit, and excessive customization is becoming a key challenge of implementing…
Prometic Bioseparations
Since 1987, Prometic Bioseparations Ltd. (PBL) has been pioneering the design, development, and manufacture of affinity purification technology for laboratory-scale and industrial-scale bioprocessing. With 30 years’ experience in the development of affinity products and design and manufacture of new custom adsorbents, PBL is a world leader in its field. PBL offers an extensive range of off-the-shelf bioseparation products for the recovery and purification of biologicals and the removal of problem contaminants. We also offer a range of semidisposable bioprocess columns…
Capturing a scFv from E. coli Using Protein L Affinity Chromatography
Single-chain variable fragments (scFv) are antibodyderived molecules with several advantages over full-length IgGs, including rapid target access and good tissue penetration. Straightforward and efficient capturing solutions similar to protein A for monoclonal antibodies (mAbs) may pave the way for the future success of this class of molecules. Herein we describe general conditions for capturing scFv from Escherichia coli with TOYOPEARL® AF-rProtein L-650F and subsequent optimization to achieve efficient purification. General Guidelines for CapturinG The Îş light chains of mAbs, Fabs,…
AbbVie: Who We Are
AbbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people, and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology, and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. Combining the focus of a biotech with the…